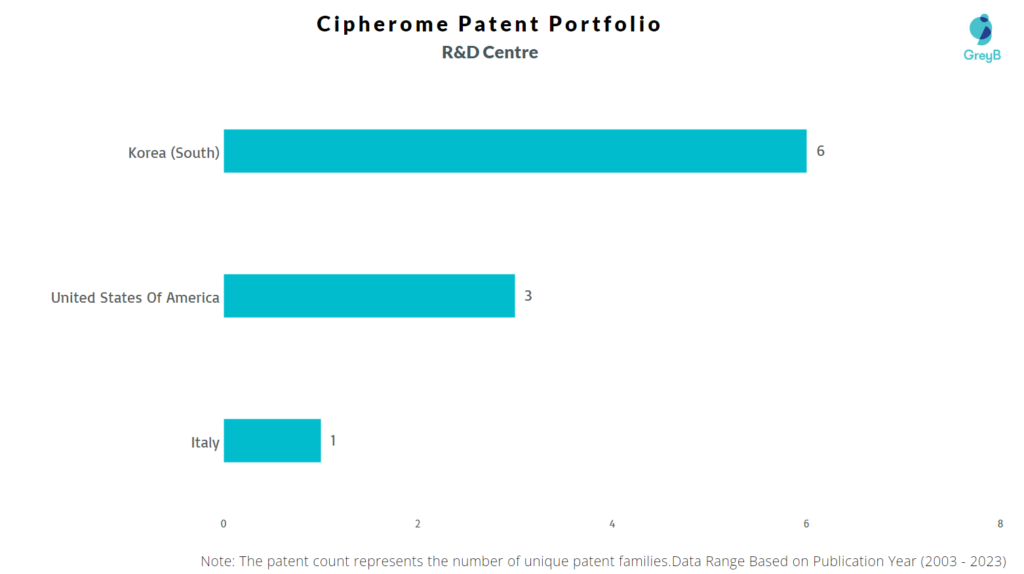
Cipherome has a total of 44 patents globally, out of which 23 have been granted. Of these 44 patents, more than 65% patents are active. The USA is where Cipherome has filed the maximum number of patents, followed by South Korea. Parallelly, South Korea seems to be the main focused R&D center of Cipherome and the United States is the origin country of the firm.
Cipherome was founded in the year 2016 by Ju Han Kim. The company provides artificial intelligence and machine learning solutions for drug related treatment.
Do read about some of the most popular patents of Cipherome which have been covered by us in this article and also you can find Cipherome patents information, the worldwide patent filing activity and its patent filing trend over the years, and many other stats over Cipherome patent portfolio.
How many patents does Cipherome have?
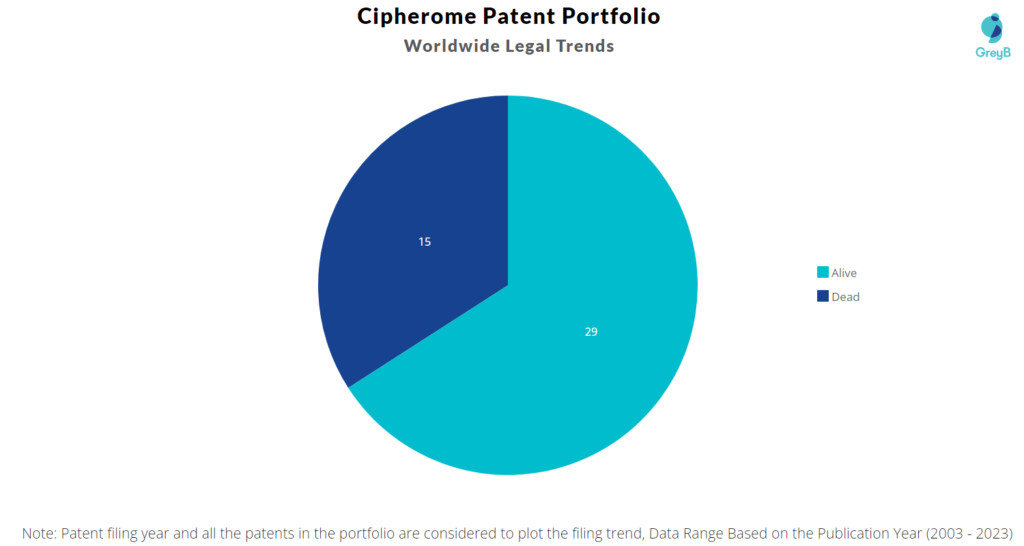
Cipherome has a total of 44 patents globally. These patents belong to 10 unique patent families. Out of 44 patents, 29 patents are active.
How Many Patents did Cipherome File Every Year?
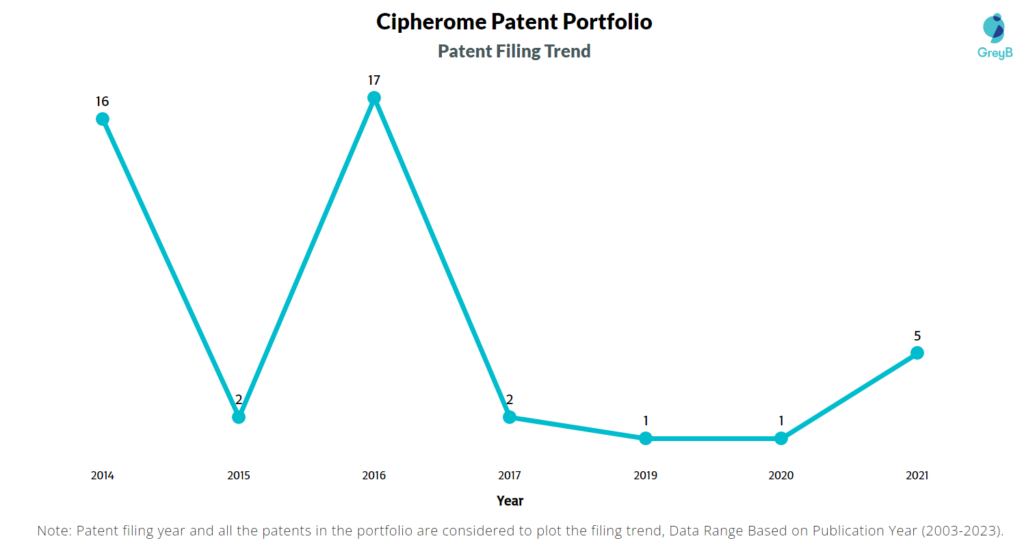
Are you wondering why there is a drop in patent filing for the last two years? It is because a patent application can take up to 18 months to get published. Certainly, it doesn’t suggest a decrease in the patent filing.
| Year of Patents Filing or Grant | Cipherome Applications Filed | Cipherome Patents Granted |
| 2022 | – | 3 |
| 2021 | 5 | 7 |
| 2020 | 1 | 4 |
| 2019 | 1 | 4 |
| 2018 | – | 1 |
| 2017 | 2 | 2 |
| 2016 | 17 | 1 |
| 2015 | 2 | 1 |
| 2014 | 16 | – |
How many Cipherome patents are Alive/Dead?
Worldwide Patents
How Many Patents did Cipherome File in Different Countries?
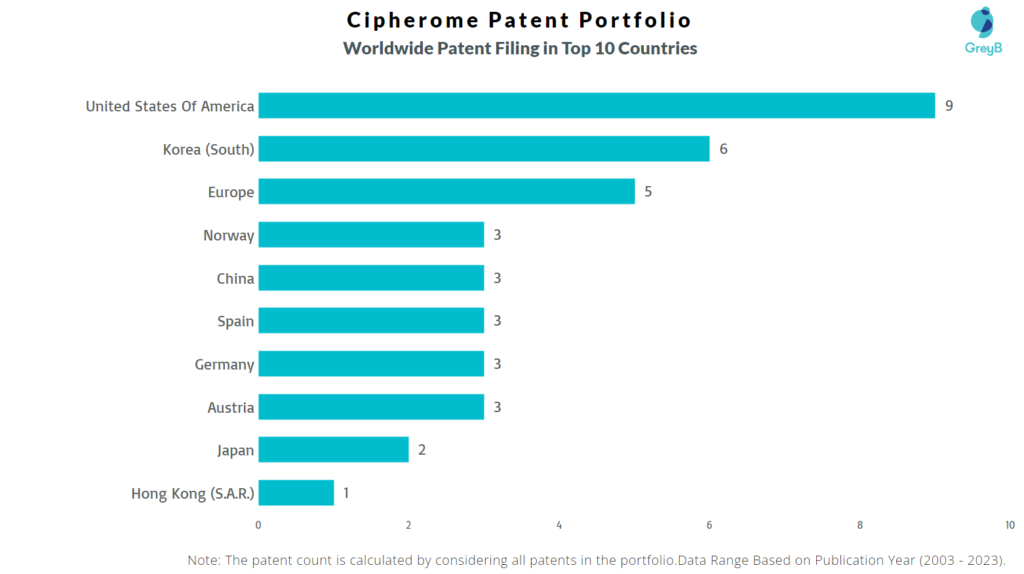
Countries in which Cipherome Filed Patents
| Country | Patents |
| United States Of America | 9 |
| Korea (South) | 6 |
| Europe | 5 |
| Norway | 3 |
| China | 3 |
| Spain | 3 |
| Germany | 3 |
| Austria | 3 |
| Japan | 2 |
| Hong Kong (S.A.R.) | 1 |
| Italy | 1 |
Where are Research Centres of Cipherome Patents Located?
Most Cited Cipherome Patent
CN109074428A is the most popular patent in the Cipherome portfolio. It has received 3 citations so far.
List of Cipherome Patents
| Cipherome Patents | Title |
| US11195594B2 | Method For Selecting Anticancer Agent Based On Protein Damage Information Of Individual To Prevent Anticancer Agent Side Effects |
| US10950326B2 | Method For Personalized Selection Of A Drug For A Subject |
| US10896743B2 | Secure Communication Of Nucleic Acid Sequence Information Through A Network |
| US20220044829A1 | Interactive User Interface For Providing Treatment Information |
| US20210327553A1 | Prediction Of Adverse Drug Reaction Based On Machine-Learned Models Using Protein Function Scores And Clinical Factors |
| US20210241849A1 | Method For Personalized Selection Of A Drug For A Subject |
| US20210104298A1 | Secure Communication Of Nucleic Acid Sequence Information Through A Network |
| EP3261008B1 | Method For Selecting Anticancer Agent Based On Protein Damage Information Of Individual To Prevent Anticancer Agent Side Effects |
| EP3495504B1 | Method And System For Selecting Drug On Basis Of Individual Protein Damage Information For Preventing Side Effects Of Drug |
| EP3037548B1 | Method And System For Selecting Drug On Basis Of Individual Protein Damage Information For Preventing Side Effects Of Drug |
| EP4136641A1 | Prediction Of Adverse Drug Reaction Based On Machine-Learned Models Using Protein Function Scores And Clinical Factors |
| CN111710434B | Methods And Systems For Determining Effects On The Function Of Proteins |
| CN105940114B | Computer Readable Medium And System For Drug Selection |
| ES2891903T3 | Method For Selecting Antineoplastic Agent Based On Protein Damage Information Of Individuals To Avoid Antineoplastic Agent Side Effects |
| DE602016063446T2 | Procedure For Selecting An Anti-Cancer Drug Based On The Individual’S Protein Damage Information To Prevent Side Effects Of The Anti-Cancer Drug |
| ES2832886T3 | Method And System For Selecting A Drug Based On Individual Protein Damage Information To Prevent Side Effects Of A Drug |
| DE602014071149T2 | Method And System For Selecting A Drug Based On Individual Protein Damage Information To Avoid The Adverse Effects Of Drug |
| ES2720999T3 | Method And System For Selecting A Drug Based On Individual Protein Damage Information To Prevent Side Effects Of A Drug |
| DE602014042981T2 | Method And System For Selecting A Drug Based On Individual Protein Damage Information To Avoid The Adverse Effects Of Drug |
| IT202000117752T2 | Method And System For Selecting A Drug On The Basis Of Individual Information Of Protein Damage For The Prevention Of Side Effects Of The Drug. |
| NO3495504B1 | Method And System For Selecting Drug On Basis Of Individual Protein Damage Information For Preventing Side Effects Of Drug |
| NO3261008B1 | Method For Selecting Anticancer Agent Based On Protein Damage Information Of Individual To Prevent Anticancer Agent Side Effects |
| NO3037548B1 | Method And System For Selecting Drug On Basis Of Individual Protein Damage Information For Preventing Side Effects Of Drug |
| KR102482818B1 | Method For Personalized Prevention Of Adverse Drug Reaction Of Osteoporosis Medication Based On Information Of Individual Deleterious Protein Sequence Variation |
| KR102482819B1 | Method For Personalized Prevention Of Adverse Drug Reaction Of Anticancer Drug Based On Information Of Individual Deleterious Protein Sequence Variation |
| JP6266110B2 | Drug Selection Method And System Based On Individual Protein Damage Information For Drug Side Effect Prevention |
| KR101788673B1 | Method For Protecting Nucleic Acid Sequence Data Security And Computer Readable Storage Medium Storing The Method |
| KR101524562B1 | Method And System For Personalized Prevention Of Adverse Drug Reaction Based On Information Of Individual Deleterious Protein Sequence Variation |
| HK40010016A1 | Method And System For Selecting Drug On Basis Of Individual Protein Damage Information For Preventing Side Effects Of Drug |
| US20180068056A1 | Method And System For Selecting Drug On Basis Of Individual Protein Damage Information For Preventing Side Effects Of Drug |
| US20170357751A1 | Computer-Implemented Evaluaton Of Drug Safety For A Population |
| EP3387570A1 | Computer-Implemented Evaluaton Of Drug Safety For A Population |
| CN109074428A | Computer-Implemented Evaluaton Of Drug Safety For A Population |
| WO2021211881A1 | Prediction Of Adverse Drug Reaction Based On Machine-Learned Models Using Protein Function Scores And Clinical Factors |
| WO2017100794A1 | Computer-Implemented Evaluaton Of Drug Safety For A Population |
| WO2016133375A1 | Method For Selecting Osteoporosis Therapeutic Agent Based On Protein Damage Information Of Individual To Prevent Osteoporosis Therapeutic Agent Side Effects |
| WO2016133374A1 | Method Of Selecting Uterine Contraction Inhibiting Agent Based On Protein Damage Information On Each Individual To Prevent Side Effects Of Uterine Contraction Inhibiting Agent |
| WO2016133373A1 | Method For Selecting Anticancer Agent Based On Protein Damage Information Of Individual To Prevent Anticancer Agent Side Effects |
| AT1428651T | Method of Selecting an Anticancer Drug Based on the Individual’s Protein Damage Information to Prevent Side Effects of the Anticancer Drug |
| AT1321212T | Method and system for selecting a drug based on individual protein damage information to avoid the side effects of drugs |
| AT1107722T | Method and system for selecting a drug based on individual protein damage information to avoid the side effects of drugs |
| JP2019505934A | Computer-Implemented Population Drug Safety Assessments |
| KR1020180124840A | Computer-Implemented Evaluaton Of Drug Safety For A Population |
| KR1020160101706A | Method For Personalized Prevention Of Adverse Drug Reaction Of Tocolytics Based On Information Of Individual Deleterious Protein Sequence Variation |
What are Cipherome’s key innovation segments?
What Technologies are Covered by Cipherome?

The chart below distributes patents filed by Cipherome
