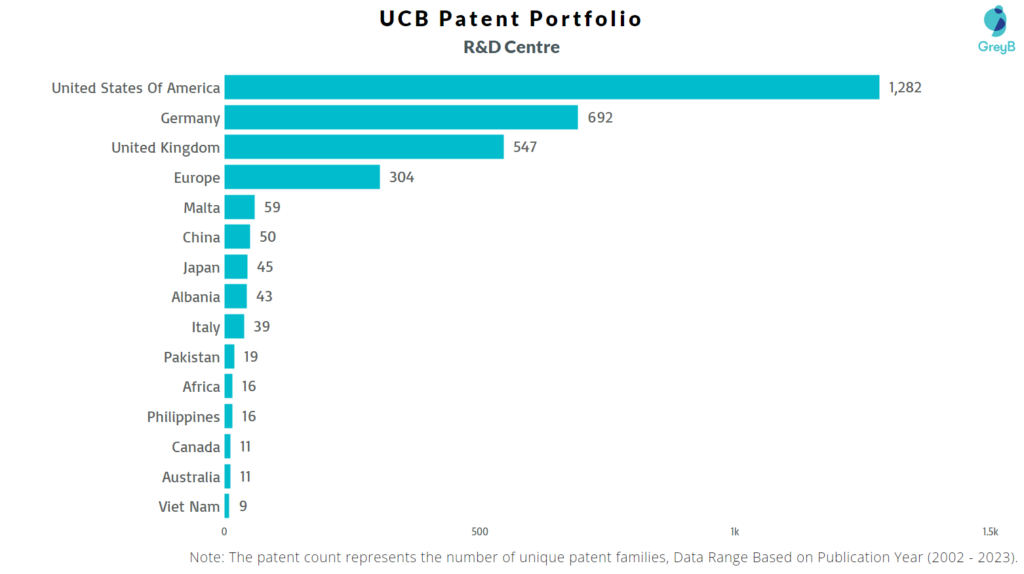
UCB has a total of 11185 patents globally, out of which 9029 have been granted. Of these 11185 patents, more than 52% patents are active. China is where UCB has filed the maximum number of patents, followed by Japan and Korea (South). Parallelly, United States of America seems to be the main focused R&D centre and also Belgium is the origin country of UCB.
UCB was founded in the year 1928. The Company operates as a biopharmaceutical company. The Company specializes in the treatment of central nervous system disorders and inflammatory diseases. As of April 2023, the market cap of UCB is $18.17 Billion.
Do read about some of the most popular patents of UCB which have been covered by us in this article and also you can find UCB patents information, the worldwide patent filing activity and its patent filing trend over the years, and many other stats over UCB patent portfolio.
Keep track of every patent move and monitor competitor activity in real-time. Click here for full insights:
How many patents does UCB have?
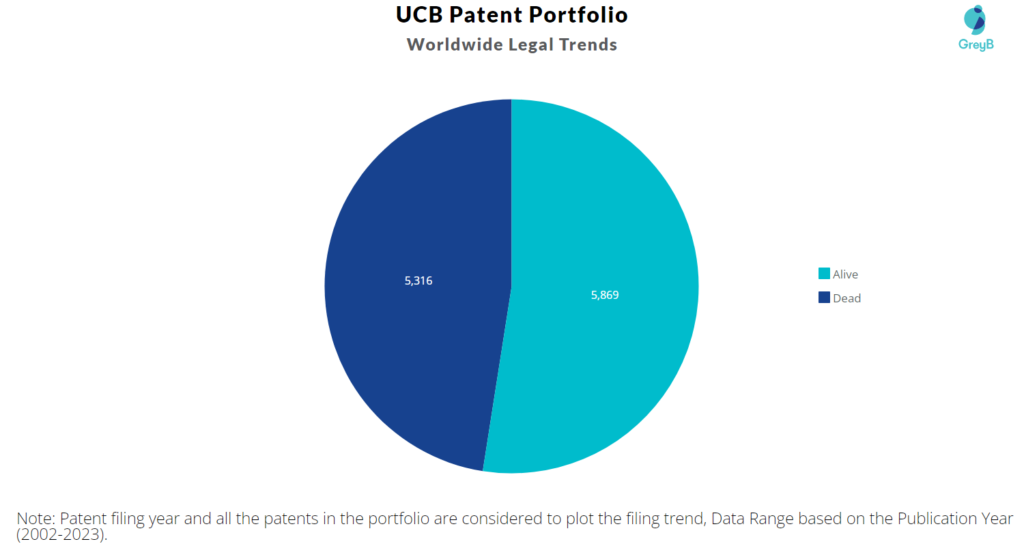
UCB has a total of 11185 patents globally. These patents belong to 3244 unique patent families. Out of 11185 patents, 5869 patents are active.
How Many Patents did UCB File Every Year?
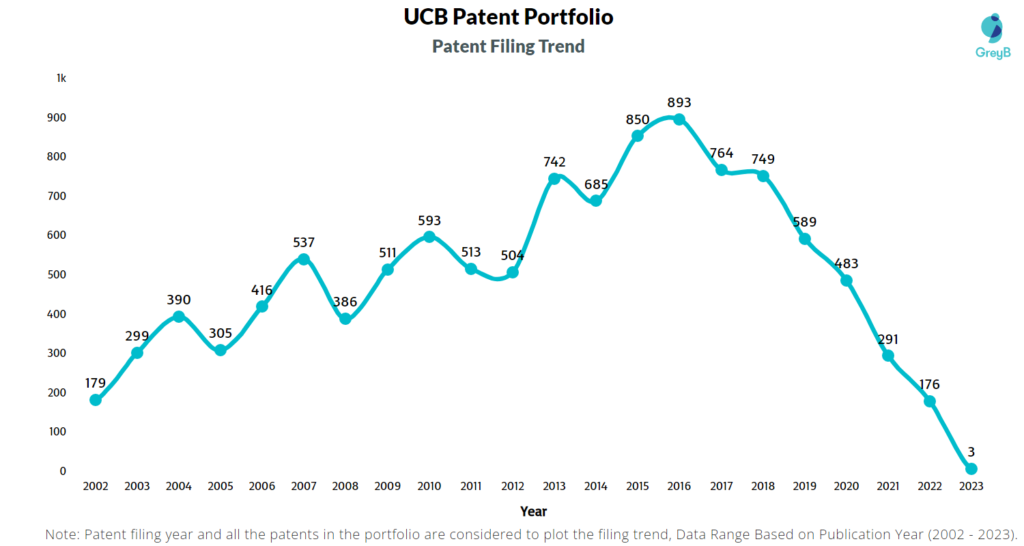
Are you wondering why there is a drop in patent filing for the last two years? It is because a patent application can take up to 18 months to get published. Certainly, it doesn’t suggest a decrease in the patent filing.
| Year of Patents Filing or Grant | UCB Applications Filed | UCB Patents Granted |
| 2023 | 3 | 105 |
| 2022 | 176 | 684 |
| 2021 | 291 | 772 |
| 2020 | 483 | 739 |
| 2019 | 589 | 971 |
| 2018 | 749 | 771 |
| 2017 | 764 | 702 |
| 2016 | 893 | 601 |
| 2015 | 850 | 496 |
| 2014 | 685 | 493 |
| 2013 | 742 | 412 |
| 2012 | 504 | 397 |
| 2011 | 513 | 473 |
Which UCB Drug Patents are Expiring in the Next 10 Years?
The patent no. US10452815B2 which is expiring in Jun, 2038, describing to manage the distribution of epilepsy medication by using a central controller with a database of patient records. The controller authorizes the first prescription based on genetic test results and schedules additional tests before approving subsequent prescriptions.
Given below is the list of few drugs patented by UCB accompanied by a patent relevant to the drug which will be available for generic drug manufacturing and usage within the next/upcoming 10 years for treatment of various diseases and disorders.
| Drug Name | Patent Number | Patent Title | Patent Expiration |
| Fintepla | US10452815B2 | Control System For Control Of Dist… | Jun, 2038 |
| Neupro | US8246980B2 | Transdermal Delivery System | Nov, 2025 |
| Nayzilam | US8217033B2 | Methods And Compositions For… | Jan, 2028 |
| Briviact | US10729653B2 | Pharmaceutical Compositions Comp… | Apr, 2030 |
| Keppra | US8802142B2 | Pharmaceutical Compositions Comp… | Dec, 2031 |
Interested in knowing about UCB’s Drug Patents Expiring in the next 10 years?
How many UCB patents are Alive/Dead?
How Many Patents did UCB File in Different Countries?
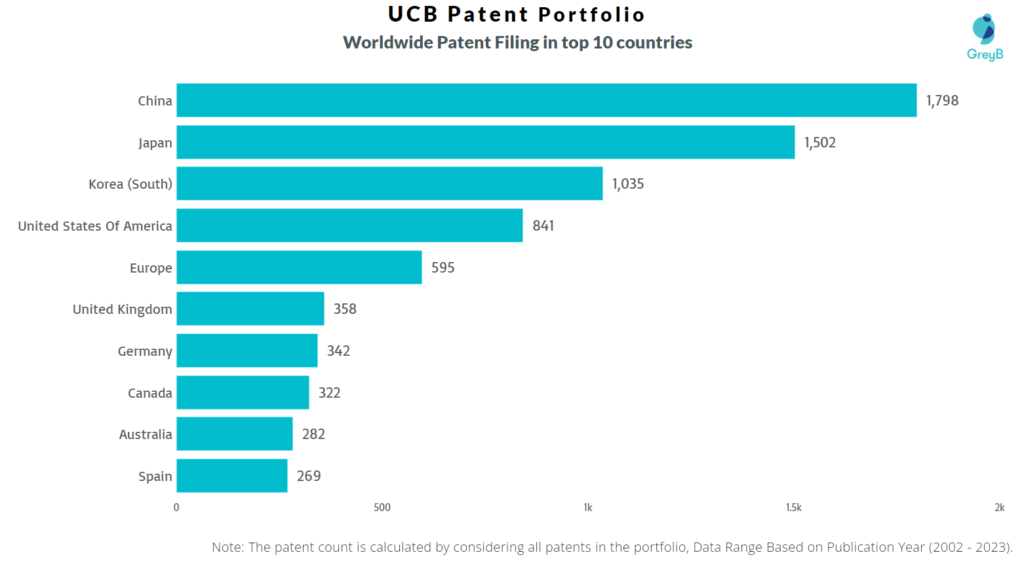
Countries in which UCB Filed Patents
| Country | Patents |
| China | 1798 |
| Japan | 1502 |
| Korea (South) | 1035 |
| United States Of America | 841 |
| Europe | 595 |
| United Kingdom | 358 |
| Germany | 342 |
| Canada | 322 |
| Australia | 282 |
| Spain | 269 |
| Brazil | 243 |
| India | 229 |
| Israel | 179 |
| Hong Kong (S.A.R.) | 178 |
| Mexico | 176 |
| Singapore | 144 |
| Eurasia | 138 |
| Denmark | 116 |
| Poland | 115 |
| Norway | 101 |
| Indonesia | 95 |
| Portugal | 94 |
| Slovenia | 85 |
| Malaysia | 84 |
| Chile | 82 |
| Taiwan | 79 |
| Russia | 77 |
| Croatia | 71 |
| Hungary | 70 |
| New Zealand | 65 |
| Colombia | 64 |
| Argentina | 63 |
| Austria | 63 |
| South Africa | 62 |
| Malta | 59 |
| Lithuania | 57 |
| Cyprus | 54 |
| Serbia | 52 |
| Philippines | 43 |
| Albania | 43 |
| Italy | 41 |
| Viet Nam | 33 |
| Peru | 29 |
| Turkey | 27 |
| Uruguay | 24 |
| Montenegro | 22 |
| Ukraine | 20 |
| Pakistan | 19 |
| Morocco | 17 |
| Africa | 16 |
| Tunisia | 14 |
| Luxembourg | 13 |
| Netherlands | 12 |
| Saudi Arabia | 9 |
| Mongolia | 7 |
| Paraguay | 7 |
| Czech Republic | 6 |
| San Marino | 6 |
| Costa Rica | 6 |
| Slovakia | 5 |
| Bulgaria | 5 |
| Gulf Cooperation Council | 5 |
| Bolivia | 4 |
| Former Yugoslav Republic of Macedonia | 4 |
| Iceland | 4 |
| Ecuador | 3 |
| Georgia | 3 |
| Uzbekistan | 3 |
| Belgium | 3 |
| Dominican Republic | 3 |
| Egypt | 2 |
| Estonia | 2 |
| United Arab Emirates | 2 |
| Kenya | 2 |
| Romania | 2 |
| Macao | 2 |
| Guatemala | 2 |
| Belarus | 1 |
| Bangladesh | 1 |
| Brunei | 1 |
| Cuba | 1 |
Where are Research Centres of UCB Patents Located?
10 Best UCB Patents
WO2009040562A1 is the most popular patent in the UCB portfolio. It has received 351 citations so far from companies like Glaxo Group Limited, MedImmune and Amgen.
Below is the list of 10 most cited patents of UCB:
| Publication Number | Citation Count |
| WO2009040562A1 | 351 |
| US6583813B1 | 331 |
| WO2013186229A1 | 313 |
| WO2010035012A1 | 309 |
| WO2014009295A1 | 274 |
| WO2014009296A1 | 241 |
| WO2011086091A1 | 213 |
| US8993731B2 | 212 |
| WO2011110621A1 | 199 |
| US7592429B2 | 173 |
How many inventions of other companies were rejected due to UCB patents?
The statistics below share strategic R&D insights. It tells the companies that were trying to protect inventions similar to UCB invention. They couldn’t because UCB had protected those before them.
Examiners at the USPTO referred 117 UCB patents in 530 rejections (35 USC § 102 or 35 USC § 103 types).
The top citing companies in the UCB patent portfolio are Amgen, Adamas Pharmaceuticals and Lts Lohmann Therapie Systeme.
List of the Companies whose Patents were rejected citing UCB –
| Company | Number of Patent Applications that faced Rejection Citing UCB Patents | Number of Rejections (102 & 103) |
| Amgen | 8 | 23 |
| Adamas Pharmaceuticals | 6 | 9 |
| Lts Lohmann Therapie Systeme | 6 | 20 |
| West Pharmaceutical Services | 5 | 8 |
| Shl Medical | 5 | 14 |
| Sanofi-Aventis Deutschland | 5 | 18 |
| Zogenix | 4 | 9 |
| Quest Diagnostics Investments | 3 | 5 |
| Individual | 3 | 4 |
| 3 | 12 |
Count of 102 and 103 Type Rejections based on UCB Patents
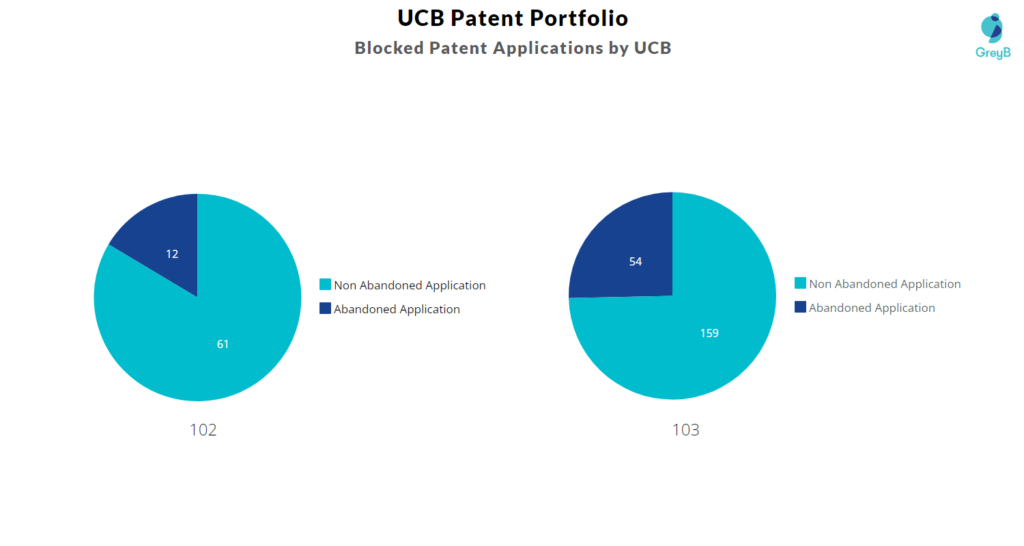
Top UCB Patents used in Rejecting Most Patent Applications
| Patent Number | Count of Rejected Applications |
| US8579866B2 | 12 |
| US6583813B1 | 10 |
| US7592429B2 | 9 |
| US7812731B2 | 8 |
| US10479824B2 | 8 |
| US9230101B2 | 8 |
| US8211462B2 | 6 |
| US7863316B2 | 6 |
| US8629246B2 | 6 |
| US8945067B2 | 6 |
| US10149818B2 | 5 |
| US7419659B2 | 5 |
| US20080274061A1 | 5 |
| US20180211010A1 | 5 |
| US7858122B2 | 5 |
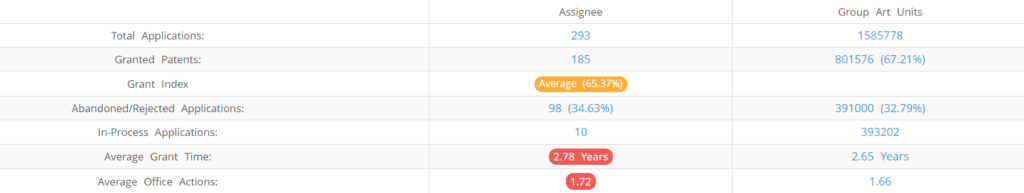
What Percentage of UCB US Patent Applications were Granted?
UCB (Excluding its subsidiaries) has filed 293 patent applications at USPTO so far (Excluding Design and PCT applications). Out of these 185 have been granted leading to a grant rate of 65.37%.
Below are the key stats of UCB patent prosecution at the USPTO.
Which Law Firms Filed Most US Patents for UCB?
| Law Firm | Total Applications | Success Rate |
| Wenderoth Lind & Ponack | 78 | 37.18% |
| Mcdonnell Boehnen Hulbert & Berghoff | 58 | 85.71% |
| Cozen O Connor | 18 | 55.56% |
| Marshall Gerstein & Borun | 15 | 93.33% |
| Morgan Lewis & Bockius | 9 | 44.44% |
| Medler Ferro Woodhouse & Mills | 8 | 83.33% |
| Saul Ewing Arnstein & Lehr | 8 | 50.00% |
| Finnegan Henderson Farabow | 6 | 66.67% |
| Cushman Darby & Cushman | 5 | 100.00% |
| Ping Wang | 5 | 80.00% |
