Starton Therapeutics has a total of 44 patents globally, out of which 1 has been granted. Of these 44 patents, more than 84% patents are active. The United States of America is where Starton Therapeutics has filed the maximum number of patents, followed by Australia and Canada. Parallelly, United States of America seems to be the main focused R&D centre and also is the origin country of Starton Therapeutics.
Starton Therapeutics was founded in 2017. Starton Therapeutics is a biotechnology business aiming to using patented continuous delivery technology to transform standard of care medicines so that cancer patients can get therapy continuously and live longer, better lives.
Do read about some of the most popular patents of Starton Therapeutics which have been covered by us in this article and also you can find Starton Therapeutics patents information, the worldwide patent filing activity and its patent filing trend over the years, and many other stats over Starton Therapeutics patent portfolio.
How many patents does Starton Therapeutics have?
Starton Therapeutics has a total of 44 patents globally. These patents belong to 7 unique patent families. Out of 44 patents, 37 patents are active.
How Many Patents did Starton Therapeutics File Every Year?

Are you wondering why there is a drop in patent filing for the last two years? It is because a patent application can take up to 18 months to get published. Certainly, it doesn’t suggest a decrease in the patent filing.
| Year of Patents Filing or Grant | Starton Therapeutics Applications Filed | Starton Therapeutics Patents Granted |
| 2023 | 2 | – |
| 2022 | 12 | – |
| 2021 | 15 | 1 |
| 2020 | 12 | – |
| 2019 | 3 | – |
How many Starton Therapeutics patents are Alive/Dead?
Worldwide Patents

How Many Patents did Starton Therapeutics File in Different Countries?
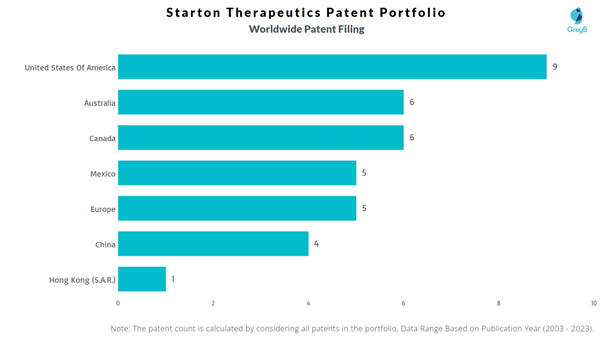
Countries in which Starton Therapeutics Filed Patents
| Country | Patent |
| United States Of America | 9 |
| Australia | 6 |
| Canada | 6 |
| Mexico | 5 |
| Europe | 5 |
| China | 4 |
| Hong Kong (S.A.R.) | 1 |
Where are Research Centers of Starton Therapeutics Patents Located?
The Research Centers of StartonTherapeutics Patents is the United States of America.
10 Best Starton Therapeutics Patents
US7467202B2 is the most popular patent in the Starton Therapeutics portfolio and it has received 2 citations.
Below is the list of 10 most cited patents of Starton Therapeutics:
| Publication Number | Citation Count |
| US11197852B2 | 2 |
| CN114901287A | 2 |
| WO2020219470A1 | 2 |
| WO2020131915A1 | 2 |
| US20220395468A1 | 1 |
| US20220218687A1 | 1 |
| US20220040194A1 | 1 |
| US20210244679A1 | 1 |
| WO2022066987A1 | 1 |
| WO2021146309A1 | 1 |
What Percentage of Starton Therapeutics US Patent Applications Were Granted?
Starton Therapeutics (Excluding its subsidiaries) has filed 9 patent applications at USPTO so far (Excluding Design and PCT applications). Out of these 6 have been granted leading to a grant rate of 100%.
Below are the key stats of Starton Therapeutics patent prosecution at the USPTO.

Which Law Firms Filed Most US Patents for Starton Therapeutics?
| Law Firm | Total Applications | Success Rate |
| Dority & Manning PA | 6 | 100% |
| Potomac Law Group Pllc | 3 | 0% |
List of Starton Therapeutics patents
| Starton Therapeutics Patents | Title |
| US11197852B2 | Continuous Delivery Of Lenalidomide And Other Immunomodulatory Agents |
| US20220395468A1 | Transdermal Drug Delivery Systems For Administration Of A Therapeutically Effective Amount Of Lenalidomide And Other Immunomodulatory Agents |
| US20220218687A1 | Stable Solutions Of Immunomodulatory Imide Compounds For Parenteral Use |
| US20220054473A1 | Continuous Delivery Of Lenalidomide And Other Immunomodulatory Agents |
| US20220040194A1 | Use Of Olanzapine For Treatment Of Parp-Inhibitor-Induced Nausea |
| US20210244679A1 | Treatment Of Vomiting And Nausea With Minimum Dose Of Olanzapine |
| US20210236417A1 | Transdermal Delivery Of Dronabinol |
| US20210220325A1 | Transdermal Delivery Of Dronabinol |
| US20210100737A1 | Transdermal Delivery Of Dronabinol |
| EP4251131A1 | Stable Solutions Of Immunomodulatory Imide Compounds For Parenteral Use |
| EP4216959A1 | Treatment Of Vomiting And Nausea With Minimum Dose Of Olanzapine |
| EP4090333A1 | Treatment Of Vomiting And Nausea With Minimum Dose Of Olanzapine |
| EP4037663A1 | Transdermal Delivery Of Dronabinol |
| EP3958866A1 | Continuous Delivery Of Lenalidomide And Other Immunomodulatory Agents |
| CN116209428A | Treatment Of Vomiting And Nausea With Minimum Doses Of Olanzapine |
| CN114901287A | Treatment Of Vomiting And Nausea With Minimum Doses Of Olanzapine |
| CN114650809A | Transdermal Delivery Of Drocannabinol |
| CN113747896A | Continuous Delivery Of Lenalidomide And Other Immunomodulatory Agents |
| WO2022260940A1 | Transdermal Drug Delivery Systems For Administration Of A Therapeutically Effective Amount Of Lenalidomide And Other Immunomodulatory Agents |
| CA3215676A1 | Transdermal Drug Delivery Systems For Administration Of A Therapeutically Effective Amount Of Lenalidomide And Other Immunomodulatory Agents |
| CA3204385A1 | Stable Solutions Of Immunomodulatory Imide Compounds For Parenteral Use |
| CA3193237A1 | Treatment Of Vomiting And Nausea With Minimum Dose Of Olanzapine |
| CA3156890A1 | Treatment Of Vomiting And Nausea With Minimum Dose Of Olanzapine |
| CA3156257A1 | Transdermal Delivery Of Dronabinol |
| CA3137594A1 | Continuous Delivery Of Lenalidomide And Other Immunomodulatory Agents |
| AU2022288865A1 | Transdermal Drug Delivery Systems For Administration Of A Therapeutically Effective Amount Of Lenalidomide And Other Immunomodulatory Agents |
| AU2022206272A1 | Stable Solutions Of Immunomodulatory Imide Compounds For Parenteral Use |
| AU2021349936A1 | Treatment Of Vomiting And Nausea With Minimum Dose Of Olanzapine |
| AU2021207844A1 | Treatment Of Vomiting And Nausea With Minimum Dose Of Olanzapine |
| AU2020358869A1 | Transdermal Delivery Of Dronabinol |
| AU2020263285A1 | Continuous Delivery Of Lenalidomide And Other Immunomodulatory Agents |
| MX2023008091A | Stable Solutions Of Immunomodulatory Compounds For Parenteral Use |
| MX2023003350A | Treatment For Vomiting And Nausea With A Minimal Dose Of Olanzapine |
| MX2022004033A | Transdermal Delivery Of Dronabinol. |
| MX2022005624A | Treatment Of Vomiting And Nausea With Minimum Dose Of Olanzapine. |
| HK40060778A | Continuous Delivery Of Lenalidomide And Other Immunomodulatory Agents |
| MX2021012901A | Continuous Delivery Of Lenalidomide And Other Immunomodulatory Agents. |
| WO2022150561A1 | Stable Solutions Of Immunomodulatory Imide Compounds For Parenteral Use |
| WO2022066987A1 | Treatment Of Vomiting And Nausea With Minimum Dose Of Olanzapine |
| WO2021146309A1 | Treatment Of Vomiting And Nausea With Minimum Dose Of Olanzapine |
| WO2021067806A1 | Transdermal Delivery Of Dronabinol |
| WO2020219470A1 | Continuous Delivery Of Lenalidomide And Other Immunomodulatory Agents |
| WO2020131915A1 | Use Of Olanzapine For Treatment Of Parp-Inhibitor-Induced Nausea |
| WO2020118091A1 | Ondansetron In-Adhesive Transdermal Patch |
What are Starton Therapeutics key innovation segments?
What Technologies are Covered by Starton Therapeutics?

The chart below distributes patents filed by Starton Therapeutics
