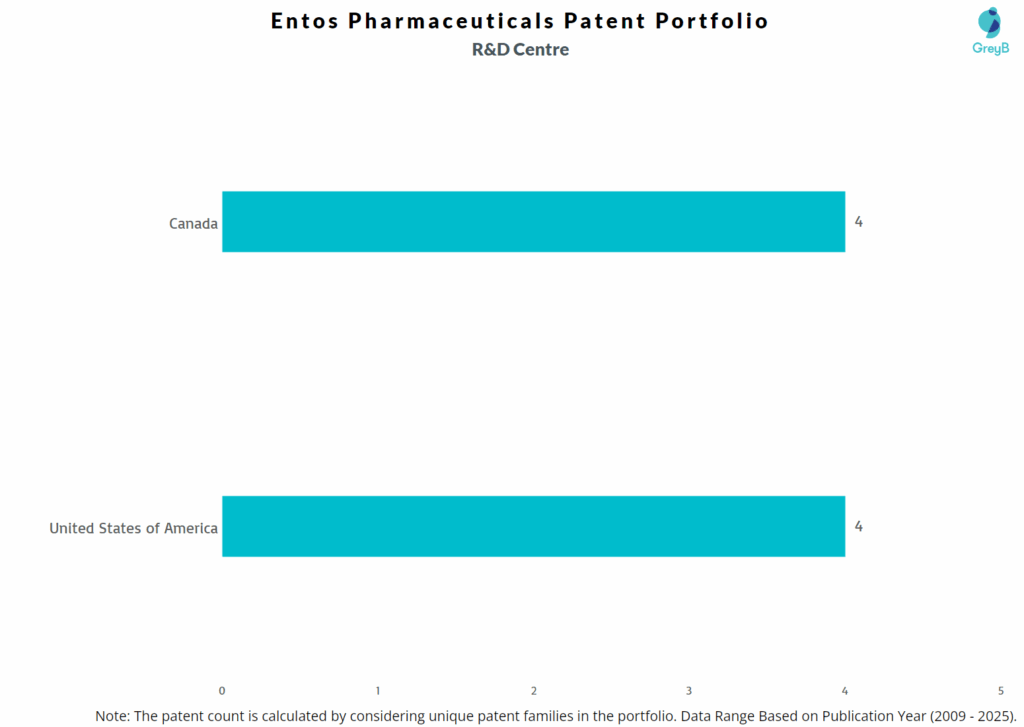
Entos Pharmaceuticals has a total of 49 patents globally, out of which 12 have been granted. Of these 49 patents, more than 71% patents are active. United States of America is where Entos Pharmaceuticals has filed the maximum number of patents, followed Canada and Australia. Parallelly, Canada seems to be the main focused R&D centre and also is the origin country of Entos Pharmaceuticals.
Entos Pharmaceuticals founded in 2016, Entos Pharmaceuticals is a clinical-stage company focused on developing next-generation genetic medicines. Using its proprietary Fusogenix proteolipid vehicle (PLV) system, the company delivers nucleic acids directly into target cells through fusion, enabling safe, effective, and redosable therapies. Entos is advancing genetic medicine with non-toxic, innovative drug delivery technologies for a range of medical applications.
Do read about some of the most popular patents of Entos Pharmaceuticals which have been covered by us in this article and also you can find Entos Pharmaceuticals patents information, the worldwide patent filing activity and its patent filing trend over the years, and many other stats over Entos Pharmaceuticals patent portfolio.
How many patents does Entos Pharmaceuticals have?
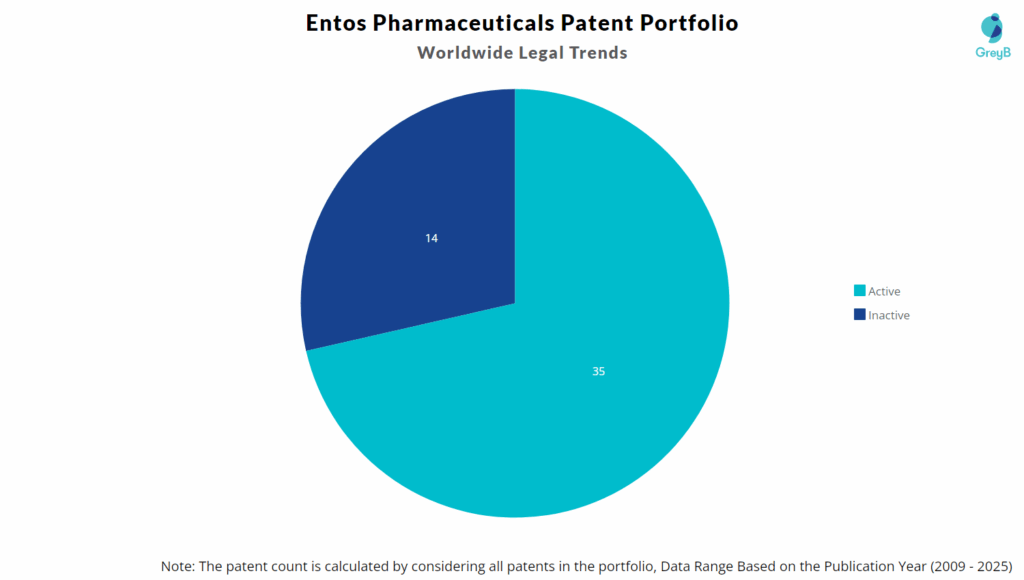
Entos Pharmaceuticals has a total of 49 patents globally. These patents belong to 8 unique patent families. Out of 49 patents, 35 patents are active.
How Many Patents did Entos Pharmaceuticals File Every Year?
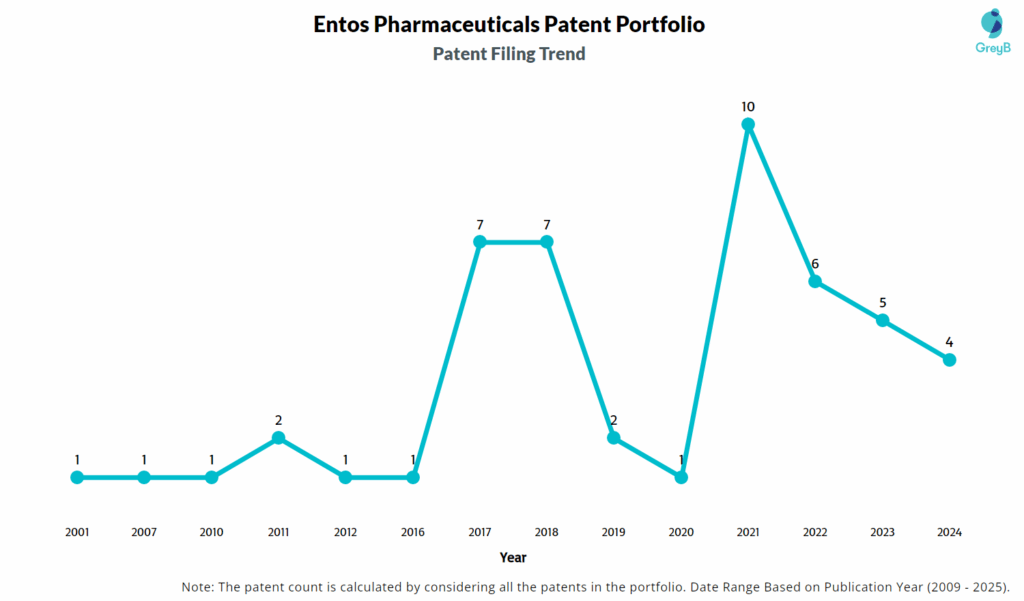
Are you wondering why there is a drop in patent filing for the last two years? It is because a patent application can take up to 18 months to get published. Certainly, it doesn’t suggest a decrease in the patent filing.
| Year of Patents Filing or Grant | Entos Pharmaceuticals Applications Filed | Entos Pharmaceuticals Patents Granted |
| 2025 | – | 2 |
| 2024 | 4 | 1 |
| 2023 | 5 | 2 |
| 2022 | 6 | 3 |
| 2021 | 10 | – |
| 2020 | 1 | – |
| 2019 | 2 | 1 |
| 2018 | 7 | – |
| 2017 | 7 | – |
| 2016 | 1 | – |
How many Entos Pharmaceuticals patents are Alive/Dead?
Worldwide Patents
How Many Patents did Entos Pharmaceuticals File in Different Countries?
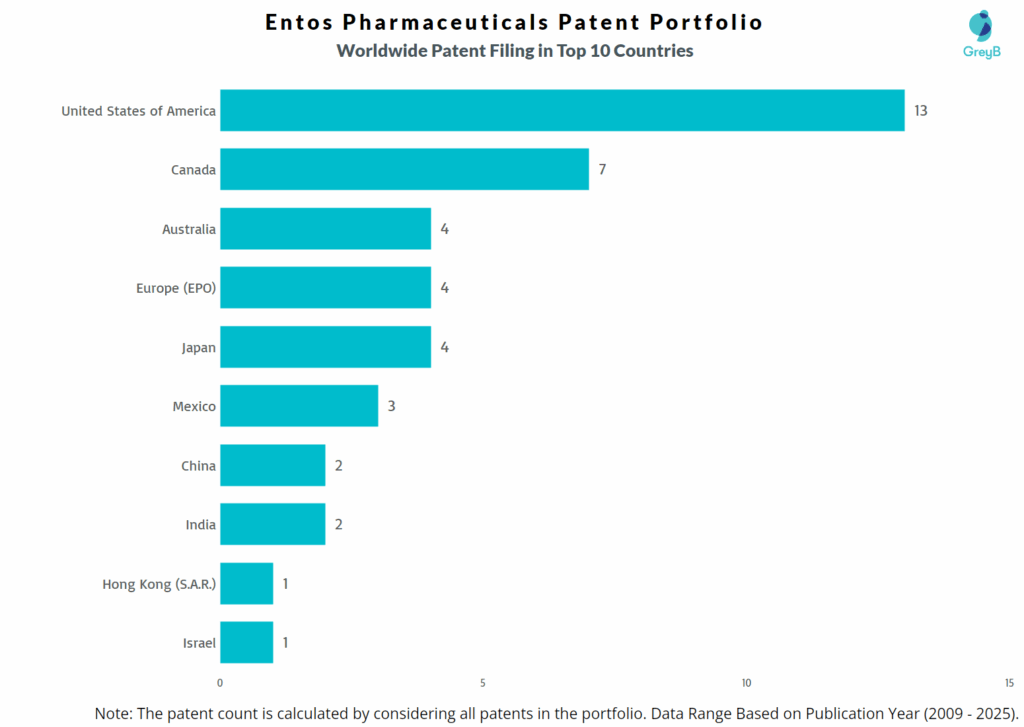
Countries in which Entos Pharmaceuticals Filed Patents
| Country | Patents |
| United States of America | 13 |
| Canada | 7 |
| Australia | 4 |
| Europe (EPO) | 4 |
| Japan | 4 |
| Mexico | 3 |
| China | 2 |
| India | 2 |
| Hong Kong (S.A.R.) | 1 |
| Israel | 1 |
| Korea (South) | 1 |
Where are Research Centres of Entos Pharmaceuticals Patents Located?
Entos Pharmaceuticals Best Patent
US8252901B2 is the most popular patent in the Entos Pharmaceuticals portfolio. It has received 9 citations so far from companies like Flagship Pioneering Innovations V Inc and Life Biosciences Inc.
What Percentage of Entos Pharmaceuticals US Patent Applications were Granted?
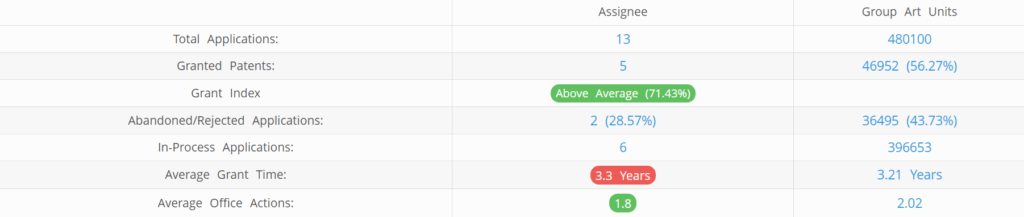
Entos Pharmaceuticals (Excluding its subsidiaries) has filed 13 patent applications at USPTO so far (Excluding Design and PCT applications). Out of these 5 have been granted leading to a grant rate of 71.43%.
Below are the key stats of Entos Pharmaceuticals patent prosecution at the USPTO.
Which Law Firms are managing US Patents for Entos Pharmaceuticals?
| Law Firm | Total Applications | Success Rate |
| Marshall Gerstein & Borun Llp | 8 | 100.00% |
| Hultquist Ip | 2 | 50.00% |
| Eversheds Sutherland US Llp | 1 | 0% |
| Gowling Wlg Canada Llp | 1 | 100.00% |
| Smart & Biggar Lp | 1 | 0.00% |
List of Genesis Therapeutics Patents
| Entos Pharmaceuticals Patents | Title |
| US12378289B2 | Recombinant Oncolytic Viruses For Treatment Of Metastatic Cancers |
| AU2018288571B2 | Recombinant Oncolytic Viruses For Treatment Of Metastatic Cancers |
| US20250090653A1 | Sars Cov-2 Vaccine, Associated Polynucleotides, And Methods Of Use |
| WO2024249651A3 | Sars Cov-2 Vaccines, Associated Polynucleotides, And Methods Of Use |
| AU2023203737B2 | Methods For Diagnosing And Treating Metastatic Cancer |
| US20240398935A1 | Sars Cov-2 Vaccines, Associated Polynucleotides, And Methods Of Use |
| US20240374520A1 | Proteolipid Vesicles Formulated With Fusion Associated Small Transmembrane Proteins |
| US20240189232A1 | Proteolipid Vesicles Formulated With Fusion Associated Small Transmembrane Proteins |
| EP4221754A4 | Proteolipid Vesicles Formulated With Fusion Associated Small Transmembrane Proteins |
| EP4440611A2 | Sars Cov-2 Vaccine, Associated Polynucleotides, And Methods Of Use |
| WO2023205239A3 | Dna Therapeutic Encoding An Antibody Or Antigen Binding Fragment |
| HK40096749A | Proteolipid Vesicles Formulated With Fusion Associated Small Transmembrane Proteins |
| AU2021351517A9 | Proteolipid Vesicles Formulated With Fusion Associated Small Transmembrane Proteins |
| AU2017285726B2 | Methods For Diagnosing And Treating Metastatic Cancer |
| JP7306829B2 | Method For Diagnosis And Treatment Of Metastatic Cancer |
| US20230192783A1 | Recombinant Polypeptides For Membrane Fusion And Uses Thereof |
| CN116685331A | Protein Lipid Vesicles Formulated With Fusion-Related Small Transmembrane Proteins |
| WO2023056070A3 | Compositions And Methods For Liver-Specific Expression Of Follistatin |
| WO2023097102A3 | Sars Cov-2 Vaccine, Associated Polynucleotides, And Methods Of Use |
| IN202327030492A | Proteolipid Vesicles Formulated With Fusion Associated Small Transmembrane Proteins |
| MX2023003774A | Proteolipid Vesicles Formulated With Fusion Associated Small Transmembrane Proteins. |
| IL301848A | Proteolipid Vesicles Formulated With Fusion Associated Small Transmembrane Proteins |
| CA3238159A1 | Sars Cov-2 Vaccine, Associated Polynucleotides, And Methods Of Use |
| JP2023543623A | Proteolipid Vesicles Loaded With Fusion-Related Transmembrane Proteins |
| JP2023123748A | Methods For Diagnosing And Treating Metastatic Cancer |
| KR1020230082033A | Proteolipid Vesicles Formulated With Fusion-Associated Small Transmembrane Proteins |
| US11459362B2 | Recombinant Polypepties For Membrane Fusion And Uses Thereof |
| US11236331B2 | Methods For Diagnosing And Treating Metastatic Cancer |
| MX396032B | Inhibitor For The Prevention And Diagnosis Of Metastatic Cancer |
| US20220112498A1 | Methods For Diagnosing And Treating Metastatic Cancer |
| WO2022067446A1 | Proteolipid Vesicles Formulated With Fusion Associated Small Transmembrane Proteins |
| MX2022012073A | Methods For Diagnosing And Treating Metastatic Cancer. |
| CA3194553A1 | Proteolipid Vesicles Formulated With Fusion Associated Small Transmembrane Proteins |
| CA3094859A1 | Proteolipid Vesicles Formulated With Fusion Associated Small Transmembrane Proteins |
| EP3642335A4 | Recombinant Oncolytic Viruses For Treatment Of Metastatic Cancers |
| EP3468564A4 | Methods For Diagnosing And Treating Metastatic Cancer |
| IN201927001167A | Methods For Diagnosing And Treating Metastatic Cancer |
| US10227386B2 | Recombinant Polypeptides For Membrane Fusion And Uses Thereof |
| CN109562121A | Methods For Diagnosing And Treating Metastatic Cancer |
| WO2018232523A1 | Recombinant Oncolytic Viruses For Treatment Of Metastatic Cancers |
| CA3068340A1 | Recombinant Oncolytic Viruses For Treatment Of Metastatic Cancers |
| WO2017214726A1 | Methods For Diagnosing And Treating Metastatic Cancer |
| CA3027430A1 | Methods For Diagnosing And Treating Metastatic Cancer |
| CA2932910A1 | Methods For Diagnosing And Treating Metastatic Cancer |
| US8252901B2 | Membrane Fusion Proteins Derived From Reovirus |
| US20120322156A1 | Membrane Fusion Proteins Derived From Reovirus |
| CA2813300A1 | Recombinant Polypeptides For Membrane Fusion And Uses Thereof |
| US7851595B2 | Membrane Fusion Proteins Derived From Reovirus |
| JP4350374B2 | Reovirus-Derived Membrane Fusion Protein |
What are Entos Pharmaceuticals key innovation segments?
What Technologies are Covered by Entos Pharmaceuticals?

The chart below distributes patents filed by Entos Pharmaceuticals in different countries on the basis of the technology protected in patents. It also represents the markets where Entos Pharmaceuticals thinks it’s important to protect particular technological inventions.
R&D Focus: How has Entos Pharmaceuticals search focus changed over the years?

EXCLUSIVE INSIGHTS COMING SOON!
Interested in knowing about the areas of innovation that are being protected by Entos Pharmaceuticals?


