Olestra (sucrose polyester), developed as a non-absorbable fat substitute, remains unapproved in the European Union due to safety, nutritional bioavailability, and gastrointestinal tolerance concerns. EFSA has not authorized its use under Regulation (EC) 1333/2008, citing insufficient evidence regarding long-term impacts on fat-soluble vitamin absorption and microbiome interactions.
As a result, food and nutraceutical manufacturers operating in EU markets require functionally equivalent, regulation-compliant lipid-replacement systems capable of delivering fat-like mouthfeel, lubrication, and stability without the metabolic and nutrient-partitioning drawbacks associated with Olestra.
This report provides a comprehensive evaluation of scientifically validated substitutes spanning lipid chemistry, carbohydrate rheology, protein colloids, and structured oleogel systems, integrating supplier-verified technical specifications and regulatory status.
Why Replace This Ingredient
Olestra’s lack of approval in Europe stems from concerns about interference with absorption of vitamins A, D, E, and K, potential impacts on carotenoid bioavailability, and gastrointestinal effects observed in dose-response modeling.
Additionally, sucrose polyesters present formulation challenges under EU clean-label and nutritional frameworks, given their non-digestible nature and requirement for mandatory nutrient fortification in some jurisdictions.
Industry reformulation pressures-ranging from fat reduction targets to sensory parity with full-fat products-necessitate replacement ingredients demonstrating stable emulsification, thermal robustness in frying and baking, oxidative resilience, and compliant nutritional positioning. Modern fat mimetics must also align with sustainability expectations, minimizing reliance on palm-derived feedstocks and enabling traceable, audited supply chains.
Regulatory Landscape Table
| Region / Authority | Status of Olestra | Key Regulatory Notes |
| EU / EFSA | Not approved | No authorization under Reg. 1333/2008; concerns on nutrient malabsorption and GI tolerance. |
| UK / FSA | Not permitted | Aligns with EU stance; no existing food additive approval. |
| USA / FDA | Approved with conditions | Allowed in savory snacks; requires fat-soluble vitamin fortification. |
| Canada / Health Canada | Not approved | Safety evidence deemed insufficient. |
| Australia/New Zealand (FSANZ) | Not approved | No adoption of sucrose polyester fat replacers. |
| Gulf/Asia Markets | Mixed/Not approved | Most markets follow Codex positions deferring approval pending long-term data. |
Manufacturers of Alternatives
1. Cargill (USA/EU) – SimPure Starch-Based Fat Mimetic System
Cargill’s SimPure line consists of physically modified food-grade starches designed to replicate fat-like creaminess in sauces, dressings, and dairy analogues. Produced via thermal–mechanical modification without chemical cross-linking, the system provides shear-thinning behavior and high water-binding capacity while maintaining pH stability from 3.2–7.0.
Thermal stability is maintained up to 140 °C for retort applications, with low retrogradation risk. Solubility is native, requiring hydration at >70 °C. Typical usage ranges from 2–6% depending on viscosity targets. Approved globally under standard starch additive regulations (EFSA E1401–E1451 as applicable). Certifications available include ISO 22000, FSSC 22000, Kosher, Halal, and Non-GMO lines. Suitable for reduced-fat spreads, soups, spoonable dressings, and dairy systems.
2. IOI Loders Croklaan (Netherlands/Malaysia) – Presdura Structured Lipid Systems
Presdura is a portfolio of palm-free enzymatically interesterified fats designed to mimic the lubrication profile of conventional triglycerides at reduced caloric load. Produced under controlled lipase-catalyzed transesterification, the products exhibit melting ranges between 28–36 °C and show oxidative stability extending >18 weeks in Schaal oven tests. pH-independent performance allows cross-category use.
These lipids are fully soluble in oil matrices and partially dispersible in aqueous systems with proper emulsifiers. Dosage typically mirrors standard fats at 8–25% depending on application. Approved in the EU as standard edible fats compliant with Regulation (EU) 1333/2008. Certifications include RSPO-MB/RSPO-SG variants, Halal, Kosher, and ISO 9001. Applications include bakery, confectionery fillings, frying oils, and plant-based analogs.
3. Ingredion (Global) – N-Dulge TRM Resistant Maltodextrin System
This carbohydrate-based fat mimetic leverages resistant maltodextrin’s bulking and mouthfeel-enhancement characteristics to deliver viscosity and creaminess in reduced-fat formulations. Manufactured through enzymatic debranching and controlled hydrolysis, the system offers high solubility in cold water and pH stability between 2.5–8.0. Heat stability surpasses 150 °C, enabling frying-adjacent thermal exposures in toppings and coatings.
Dosage is typically 3–12% for bodying and 15–25% for full fat replacement in dairy desserts. EFSA classifies resistant maltodextrin as a dietary fiber with established GRAS history globally. Certifications include Non-GMO Project, Gluten-Free, FSSC 22000, and Halal/Kosher lines. Ideal for beverages, dairy analogs, soups, and nutritional bars.
4. Kerry Group (Ireland) – Biobake Enzyme-Modulated Fat Reduction Platform
Biobake employs protease- and amylase-mediated matrix modification to generate viscoelastic structures that replicate fat’s lubricity and crumb tenderness in bakery systems. Produced through controlled enzymatic treatment of wheat or alternative cereal matrices, the system creates micro-air cell stabilization and controlled water migration, enhancing softness.
Thermal stability extends through standard baking cycles up to 240 °C. pH tolerance spans 4.0–7.5. Usage levels are low (0.1–0.5% enzyme blend), though activator substrates may reach 1–3%. Approved globally within existing enzyme legislation (EU Food Enzymes Regulation 1332/2008). Kerry offers BRC and ISO audited production. Applications include breads, cakes, pastries, and fried dough systems.
5. Unilever Foods Solutions (EU) – Plant-Based Oleogel Platforms (Sunflower Wax–Oil Systems)
Unilever’s internal oleogelation technologies employ sunflower wax crystallized within high-oleic sunflower oil to simulate solid-fat functionality at reduced saturated fat content. The oleogels exhibit storage moduli (G’) consistent with plastic fats and maintain structural integrity from 4–35 °C. pH independence allows integration into acidic emulsions.
Thermal melting occurs at 55–75 °C depending on wax load. Solubility is lipid-only; emulsifiers such as PGPR or lecithin improve dispersion. Dosage ranges from 6–12% oleogel relative to fat phase. Sunflower wax is EFSA-approved under general food additive guidelines when used as processing aids or structuring agents. Certifications include ISO 22000, Kosher, and Halal. Applications span spreads, fillings, meat analogues, and bakery fats.
Comparative Technical Table
| Alternative Class | Active Compound | Mechanism of Action | Stability | EU Regulatory Status | Expected Performance |
| Modified starches | Amylopectin-rich starch | Water-binding & viscosity modulation | Heat stable to 140 °C; pH 3–7 | Approved (various E-codes) | Creaminess, bulk, viscosity |
| Resistant maltodextrin | Low-DE glucose polymers | Bulking + mouthfeel engineering | Heat stable to 150 °C; pH 2.5–8 | Approved | Mild creaminess, low kcal |
| Interesterified fats | Structured triglycerides | Controlled melting & lubrication | Oxidatively stable | Approved as fats | High sensory fidelity |
| Oleogels | Wax-structured oils | Crystalline lipid networks | Heat stable to 70 °C | Approved (structuring agents vary) | Plasticity, spreadability |
| Protein microgels | Denatured proteins | Colloid formation & lubrication | pH sensitive; heat stable post-set | Approved | Dairy-like creaminess |
Segmentation of Alternatives by Scientific Domain
1. Lipid Chemistry Substitutes
Structured triglycerides, interesterified fats, and medium-chain triglyceride blends deliver lubrication and mouthfeel more closely aligned with conventional fats than Olestra, without nutrient-absorption interference.
Their melting curves can be engineered via enzymatic interesterification to replicate plasticity in spreads or brittleness in confectionery coatings. Oxidative stability modeling suggests shelf-life enhancement when phenolic antioxidants are incorporated. EU compliance is straightforward, classifying these materials as edible oils and fats rather than novel additives.
2. Carbohydrate Rheology Systems
Modified starches, resistant maltodextrins, and hydrocolloids (e.g., xanthan, guar, CMC) provide bulk, viscosity, freeze-thaw stability, and fat-like creaminess. Mechanistically, they modulate shear-thinning behavior and water distribution, creating pseudo-fat mouthfeel without contributing caloric lipids.
These systems are thermally robust and widely approved under EU additive codes, though sensory optimization requires particle-size control and rheological tuning to prevent gumminess.
3. Protein Colloid Stabilizers
Whey protein isolate, pea protein, and micellar casein can generate heat-stable emulsions and air–fat–water multiphasic structures that emulate the lubricity and creaminess typically delivered by fats.
Protein-based fat mimetics rely on denaturation and aggregation kinetics, yielding microgels with defined fractal dimensions. pH sensitivity (notably 4.2–5.4 isoelectric zones) requires buffering or co-stabilizers. Regulatory approval is established for food proteins except where novel processing methods are used.
4. Material-Science Oleogels
Wax-, monoacylglycerol-, and phytosterol-based oleogels create viscoelastic lipid networks resembling saturated fats while delivering lower saturated fat profiles. Their melting temperatures, oil-binding capacities, and oxidative stabilities can be tailored through crystallization rates and polymorph control.
Oleogels require emulsification strategies when introduced into high-moisture matrices. EU acceptance is generally straightforward where waxes and structuring agents are approved under existing additive frameworks.
5. Hybrid Multi-Phase Systems
Emerging fat-reduction solutions combine carbohydrate gelling matrices with lipid microdroplets or oleogel inclusions to achieve multiparametric mimicry of fat’s tribology profile.
These systems enable high-fidelity replication of creaminess, coating behavior, and thermal tolerance without the nutrient malabsorption associated with Olestra. They align well with EU reformulation targets and offer scalability for dairy, bakery, and savory applications.
Replacing Olestra for food applications requires a deep understanding of its role and the properties of suitable alternatives. R&D teams have to search across hundreds of scientific journals, patents, regulatory filings, industry reports, and databases to gather comprehensive data on potential alternative ingredients.
This is where an AI-powered research tool like Slate helps out. We analyzed the alternatives, their mechanism, thermal stability, scalability, and safety insights using Slate, and all it needs is a simple query, like “What are the alternatives to Olestra for food applications?”
Within minutes, Slate scours through hundreds of patents and research papers, finds highly relevant inventions, and presents them in a neat dashboard. Here’s a screenshot as an example:
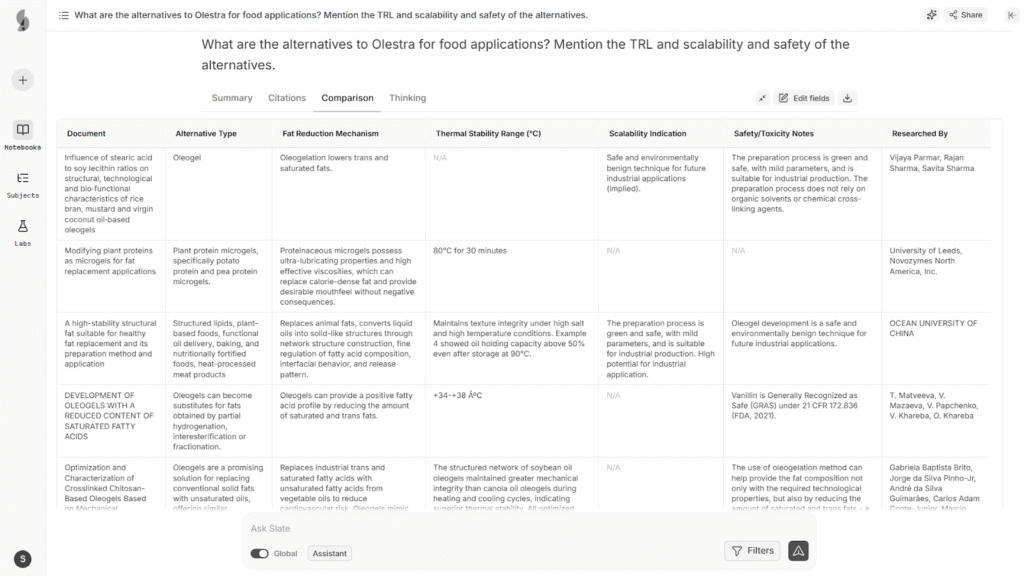
Access the full research on Olestra alternatives here.
Research Gaps & White-Space Opportunities
1. Digestive Fate & Bioaccessibility Modeling
Current in vitro digestion models insufficiently predict nutrient release, matrix interactions, and lipid droplet restructuring in reduced-fat systems.
Advanced dynamic GI simulators with mucin-layer integration are required to correlate fat-mimetic performance with real nutrient absorption.
2. High-Temperature Performance Limitations
Carbohydrate-based mimetics exhibit viscosity loss or browning at frying temperatures. There is a need for thermally stable hybrid matrices capable of preserving lubrication behavior above 180 °C without generating acrylamide precursors.
3. Multi-Functional Hybrid Systems
Few commercial solutions integrate lubricity, water management, oxidative stability, and freeze–thaw robustness simultaneously.
Research is needed to develop tunable multi-phase architectures combining oleogels, microgels, and rheology modifiers.
4. Sensory–Tribology Predictive Modeling
Despite advances in tribometers and oral processing models, correlations between instrumented friction curves and consumer-perceived creaminess remain weak.Improved AI-driven predictive models could streamline R&D trials and accelerate regulatory submissions.
5. Compatibility in High-Protein or High-Fiber Matrices
Fat mimetics often underperform in high-protein or high-fiber formulations due to competitive water binding and phase separation.
Mechanistic studies on hydration kinetics and microstructural integration are needed to avoid textural collapse or syneresis.
Formulation Considerations
Successful replacement of Olestra requires alignment of fat mimetic behavior with processing stresses, including shear rates in mixing, thermal loads in baking or pasteurization, and freeze–thaw cycles. Solubility and hydration kinetics of carbohydrate systems must be tightly controlled to prevent graininess.
Lipid systems demand antioxidant strategies (e.g., tocopherols, rosemary extract) to mitigate oxidative deterioration. pH control is critical for protein colloids, requiring buffering to avoid isoelectric destabilization. Packaging materials must offer adequate oxygen and moisture barrier properties, particularly for oleogels sensitive to autoxidation.
Dosage optimization should rely on small-scale rheological mapping (G’, G”), tribology evaluation, and sensory benchmarking against full-fat controls. Labeling implications must consider EU requirements around dietary fiber claims, fat reduction thresholds, and avoidance of misleading nutrition claims.
R&D Implementation Framework
1. Audit & Risk Mapping
Evaluate current formulations for thermal load, shear intensity, pH range, allergen presence, and nutritional claims. Identify regulatory constraints for EU market entry and risk factors associated with fat removal.
2. Screening of Alternatives
Assess multi-domain fat mimetics (lipid, carbohydrate, protein, oleogel, hybrid) using compositional, rheological, and tribological screening. Select candidates meeting sensory, stability, and regulatory benchmarks.
3. Trial Design
Construct factorial trials varying dosage, hydration, emulsification, thermal treatment, and antioxidant inclusion. Apply instrumental tribology, RVA/DSC, and microstructure imaging to quantify performance.
4. Data Integration
Integrate rheology, sensory, shelf-life, and regulatory data using multivariate models to predict scalability and long-term stability. Identify interactions requiring formulation adjustments.
5. Scale-Up
Pilot-scale validation with industrial equipment, ensuring thermal, shear, and mixing profiles match commercial lines. Conduct final regulatory compliance checks and document specifications for each fat-mimetic system.
Conclusion
Olestra’s non-approval across Europe necessitates a structured, science-driven approach to fat replacement that avoids nutrient malabsorption risks while achieving the tribological and sensory attributes of conventional lipids.
Modern alternatives-including structured lipids, carbohydrate rheology systems, protein colloids, and oleogels-offer robust, regulatory-compliant pathways for reformulation across savory, bakery, and dairy categories.
R&D teams must rely on rheological engineering, microstructure control, and multi-phase system design to ensure parity with full-fat benchmarks while maintaining EU compliance.
Regulatory Disclaimer
This document is for R&D informational purposes only and does not constitute regulatory or legal advice. Verify regional approvals, supplier specifications, and application performance data before commercialization.
Request supplier specification sheets. Download the fat-mimetic trial-design template.

