Autonomous Medical Devices has a total of 31 patents globally, out of which 9 have been granted. Of these 31 patents, more than 96% patents are active. The United States of America is where Autonomous Medical Devices has filed the maximum number of patents, followed by Europe and Canada. Parallelly, United States of America seems to be the main focused R&D centre and also is the origin country of Autonomous Medical Devices.
Autonomous Medical Devices was founded in 2013. Autonomous Medical Devices has developed a medical device specifically engineered for the detection of pathogens and viruses.
Do read about some of the most popular patents of Autonomous Medical Devices which have been covered by us in this article and also you can find Autonomous Medical Devices patents information, the worldwide patent filing activity and its patent filing trend over the years, and many other stats over Autonomous Medical Devices patent portfolio.
How many patents does Autonomous Medical Devices have?
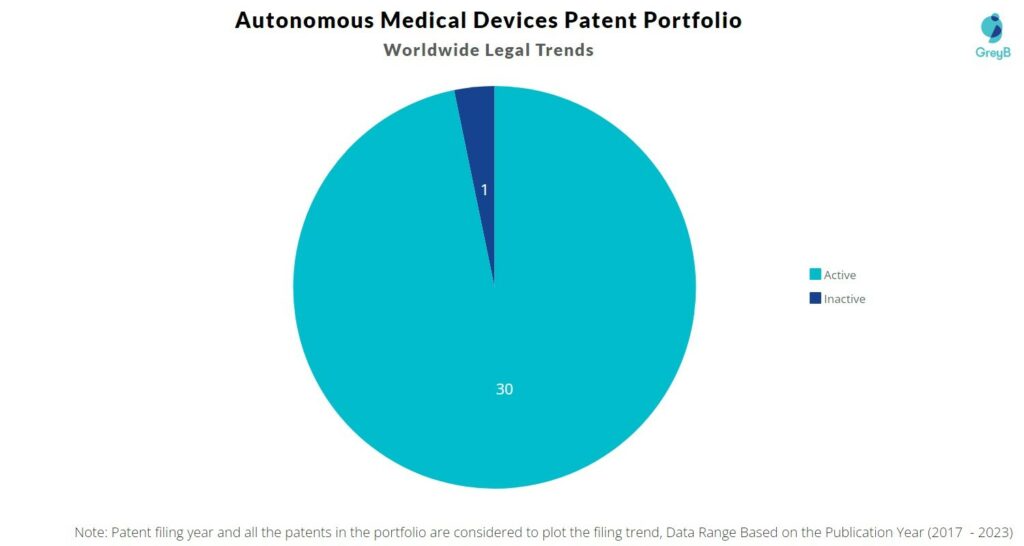
Autonomous Medical Devices has a total of 31 patents globally. These patents belong to 6 unique patent families. Out of 31 patents, 30 patents are active.
How Many Patents did Autonomous Medical Devices File Every Year?
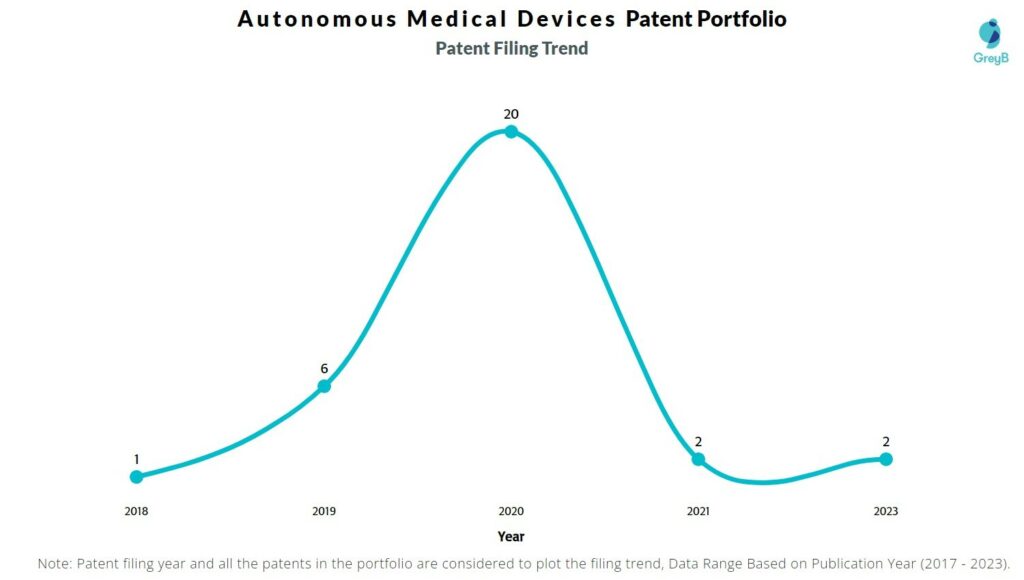
Are you wondering why there is a drop in patent filing for the last two years? It is because a patent application can take up to 18 months to get published. Certainly, it doesn’t suggest a decrease in the patent filing.
| Year of Patents Filing or Grant | Autonomous Medical Devices Applications Filed | Autonomous Medical Devices Patents Granted |
| 2023 | 2 | 5 |
| 2022 | – | 2 |
| 2021 | 2 | 2 |
| 2020 | 20 | – |
| 2019 | 6 | – |
| 2018 | 1 | – |
How many Autonomous Medical Devices patents are Alive/Dead?
Worldwide Patents
How Many Patents did Autonomous Medical Devices File in Different Countries?
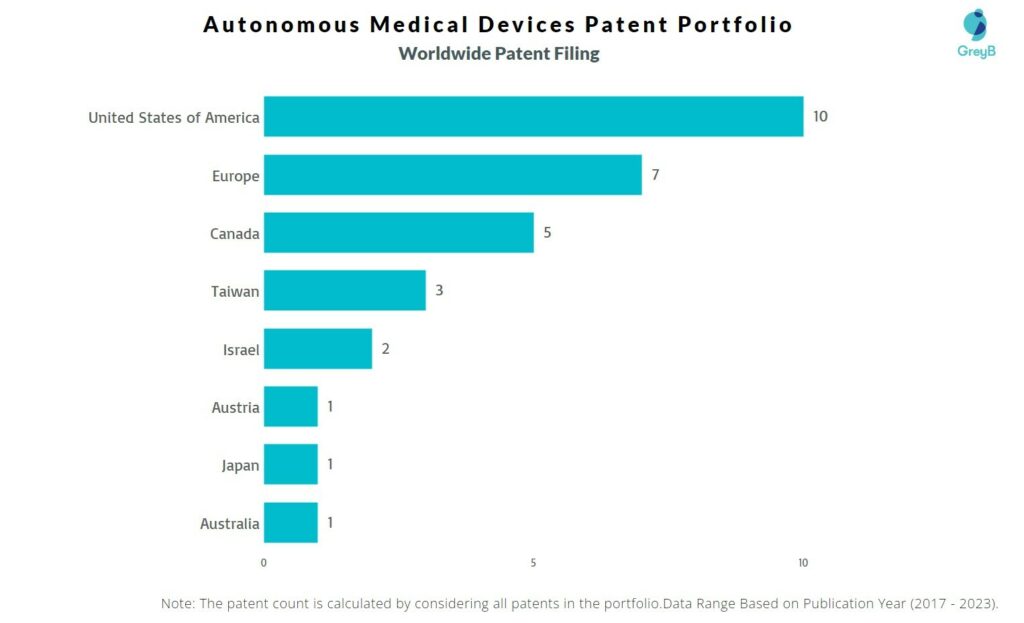
Countries in which Autonomous Medical Devices Filed Patents
| Country | Patent |
| United States of America | 10 |
| Europe | 7 |
| Canada | 5 |
| Taiwan | 3 |
| Israel | 2 |
| Austria | 1 |
| Japan | 1 |
| Australia | 1 |
Where are Research Centers of Autonomous Medical Devices Patents Located?
The Research Centers of Autonomous Medical Devices Patents is the United States of America.
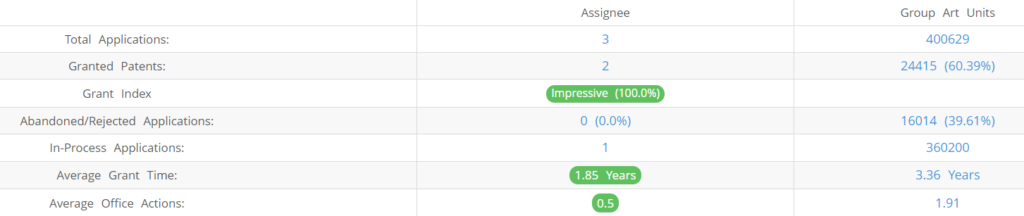
What Percentage of Autonomous Medical Devices US Patent Applications Were Granted?
Autonomous Medical Devices (Excluding its subsidiaries) has filed 3 patent applications at USPTO so far (Excluding Design and PCT applications). Out of these 2 have been granted leading to a grant rate of 100%.
Below are the key stats of Autonomous Medical Devices patent prosecution at the USPTO.
Which Law Firms Filed Most US Patents for Autonomous Medical Devices?
| Law Firm | Total Applications | Success Rate |
| Dawes Patent Law Group | 3 | 100% |
List of Autonomous Medical Devices patents
| Autonomous Medical Devices Patents | Title |
| US11567068B2 | Detection Of Cardiac Troponin Or Biological Markers Via Shear Horizontal Surface Acoustic Wave Biosensor Using A Wet-Dry Bioanalytical Technique |
| US11224874B2 | Apparatus For Automatic Sampling Of Biological Species Employing Disk Microfluidics System |
| US11130994B2 | Automated, Cloud-Based, Point-Of-Care (Poc) Pathogen And Antibody Array Detection System And Method |
| US11020741B2 | Field Portable, Handheld, Recirculating Surface Acoustic Wave And Method For Operating The Same |
| EP3948255B1 | Detection Of Cardiac Troponin Or Biological Markers Via Shear Horizontal Surface Acoustic Wave Biosensor Using A Wet-Dry Bioanalytical Technique |
| US20220097064A1 | Apparatus For Automatic Sampling Of Biological Species Employing Disk Microfluidics System |
| US20220097063A1 | Apparatus For Automatic Sampling Of Biological Species Employing Disk Microfluidics System |
| US20210402392A1 | Apparatus And Method For Point Of Care, Rapid, Field-Deployable Diagnostic Testing Of Covid-19, Viruses, Antibodies And Markers, Autolab 20 |
| US20210310044A1 | Antibody Or Aptamer Conjugated-Polynucleotides And Detection Methods And Microfluidics Devices Using The Same |
| US20210311028A1 | Antibody Or Aptamer Conjugated-Lipid Vesicles And Detection Methods And Microfluidics Devices Using Same |
| US20210293798A1 | Chelator-Coated Field Effect Transistor And Devices And Methods Using Same |
| EP3837541A4 | Chelator-Coated Field Effect Transistor And Devices And Methods Using Same |
| EP3836160A1 | An Automated, Cloud-Based, Point-Of-Care (Poc) Pathogen And Antibody Array Detection Method |
| EP3836159A1 | An Automated, Cloud-Based, Point-Of-Care (Poc) Pathogen And Antibody Array Detection System |
| EP3836152A1 | System And Method For Point-Of-Care, Rapid, Field-Deployable Diagnostic Testing Of Viruses |
| EP3835786A1 | System And Method For Point-Of-Care, Rapid, Field-Deployable Diagnostic Testing Of Viruses |
| EP3836151A1 | System And Method For Point-Of-Care, Rapid, Field-Deployable Diagnostic Testing Of Viruses |
| WO2023137020A1 | Platforms For Antibody Subpopulation Detection |
| CA3098079C | Apparatus And Method For Point-Of-Care, Rapid, Field-Deployable Diagnostic Testing Of Covid-19, Viruses, Antibodies And Markers |
| CA3198903A1 | Apparatus And Method For Point-Of-Care, Rapid, Field-Deployable Diagnostic Testing Of Covid-19, Viruses, Antibodies And Markers |
| CA3198897A1 | Apparatus And Method For Point-Of-Care, Rapid, Field-Deployable Diagnostic Testing Of Covid-19, Viruses, Antibodies And Markers |
| CA3093147A1 | An Automated, Cloud-Based, Point-Of-Care (Poc) Pathogen And Antibody Array Detection System And Method |
| CA3133568A1 | Detection Of Cardiac Troponin Or Biological Markers Via Shear Horizontal Surface Acoustic Wave Biosensor Using A Wet-Dry Bioanalytical Technique |
| TWI800768B | Apparatus And Method For Point-Of-Care, Rapid, Field-Deployable Diagnostic Testing Of Covid-19, Viruses, Antibodies And Markers – Autolab 20 |
| TWI772882B | An Automated, Cloud-Based, Point-Of-Care (Poc) Pathogen And Antibody Array Detection System And Method |
| TW202342499A | Platforms For Antibody Subpopulation Detection |
| AT1576585T | Detection of cardiac troponin or biological markers by biosensor with shear horizontal acoustic waves using a bioanalytical wet-dry technique |
| IL277915A | An Automated, Cloud-Based, Point-Of-Care (Poc) Pathogen And Antibody Array Detection System And Method |
| IL278697A | Apparatus And Method For Point-Of-Care, Rapid, Field-Deployable Diagnostic Testing Of Covid-19, Viruses, Antibodies And Markers |
| AU2020245058B2 | Detection Of Cardiac Troponin Or Biological Markers Via Shear Horizontal Surface Acoustic Wave Biosensor Using A Wet-Dry Bioanalytical Technique |
| JP2022527989A | Detection Of Cardiac Troponin Or Biological Markers By Shear Wave Surface Acoustic Wave Biosensors Using Wet And Dry Biochemical Analysis Techniques |
What Technologies are Covered by Autonomous Medical Devices?

The chart below distributes patents filed by Autonomous Medical Devices
