Centivax has a total of 31 patents globally, out of which 10 have been granted. Of these 31 patents, more than 67% patents are active. The United States of America is where Centivax has filed the maximum number of patents, followed by Europe and Hong Kong (S.A.R.). Parallelly, United States of America seems to be the main focused R&D centre and also is the origin country of Centivax.
Centivax was founded in 2019. Centivax is a company focused on developing treatments to combat quickly changing viruses. They use advanced technology to create antibodies that target different strains of viruses, including SARS-CoV-2. This helps in developing vaccines to protect people from getting sick.
Do read about some of the most popular patents of Centivax which have been covered by us in this article and also you can find Centivax patents information, the worldwide patent filing activity and its patent filing trend over the years, and many other stats over Centivax patent portfolio.
How many patents does Centivax have?
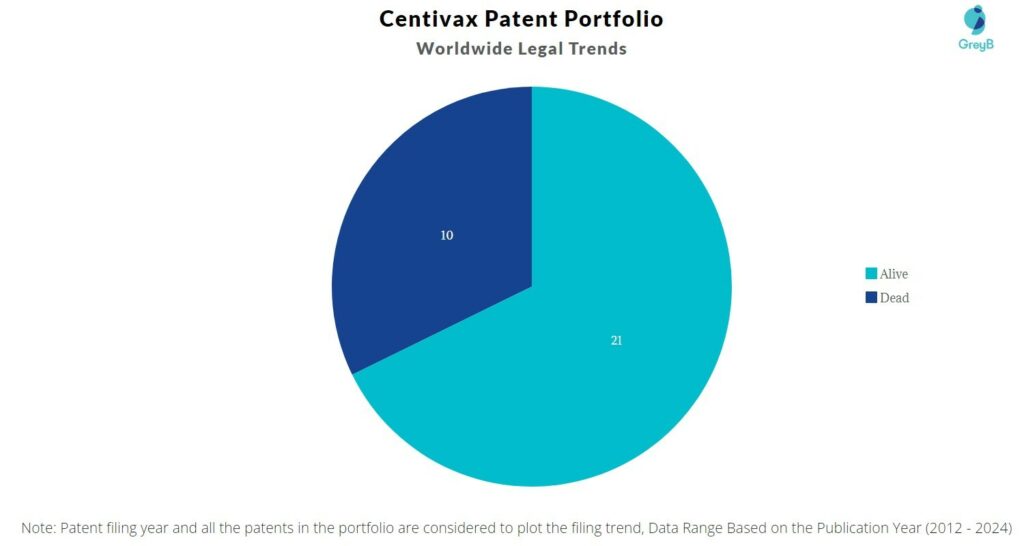
Centivax has a total of 31 patents globally. These patents belong to 7 unique patent families. Out of 31 patents, 21 patents are active.
How Many Patents did Centivax File Every Year?
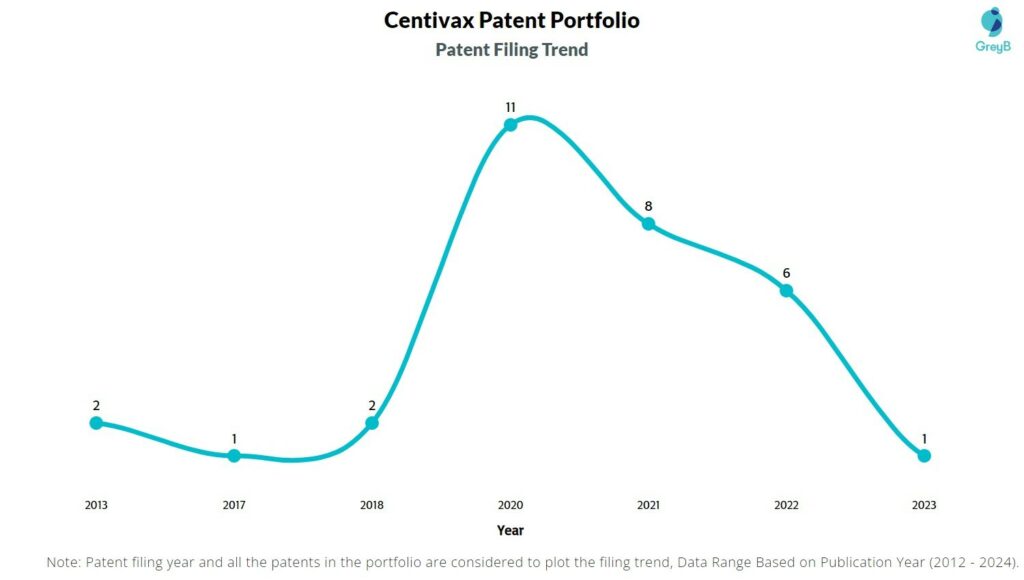
Are you wondering why there is a drop in patent filing for the last two years? It is because a patent application can take up to 18 months to get published. Certainly, it doesn’t suggest a decrease in the patent filing.
| Year of Patents Filing or Grant | Centivax Applications Filed | Centivax Patents Granted |
| 2023 | 1 | 1 |
| 2022 | 6 | – |
| 2021 | 8 | 6 |
| 2020 | 11 | 1 |
| 2019 | – | 1 |
| 2018 | 2 | 1 |
| 2017 | 1 | – |
| 2013 | 2 | – |
How many Centivax patents are Alive/Dead?
Worldwide Patents
How Many Patents did Centivax File in Different Countries?
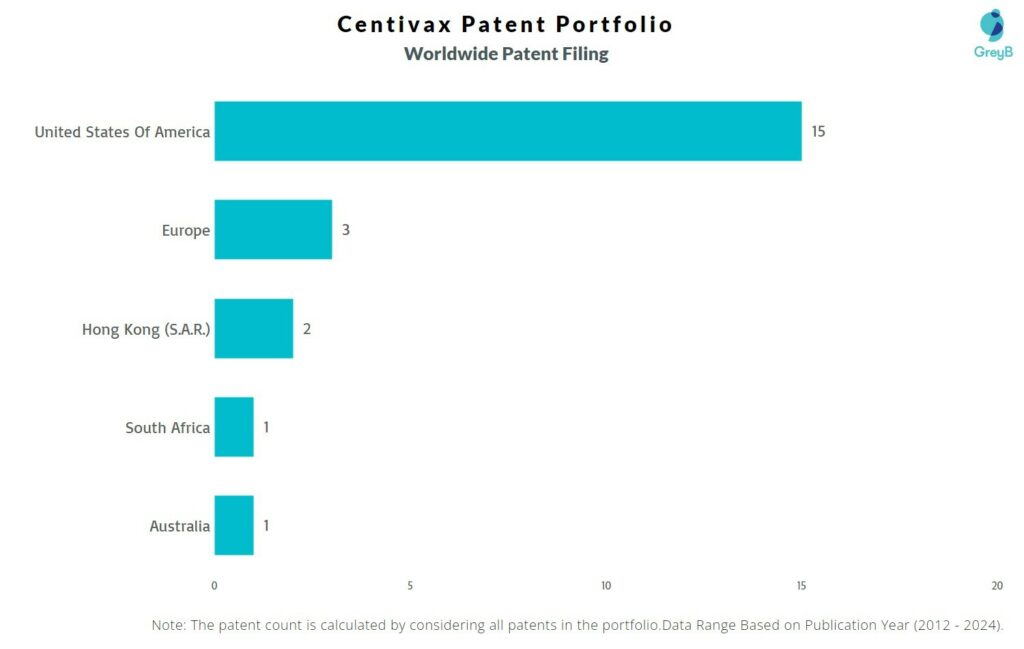
Countries in which Centivax Filed Patents
| Country | Patent |
| United States Of America | 15 |
| Europe | 3 |
| Hong Kong (S.A.R.) | 2 |
| South Africa | 1 |
| Australia | 1 |
Where are Research Centers of Centivax Patents Located?
The Research Centers of Centivax Patents is the United States of America.
10 Best Centivax Patents
US11021531B1 is the most popular patent in the Centivax portfolio. It has received 7 citations so far from companies like Bio-Rad Laboratories, Regeneron Pharmaceuticals, Guangzhou Bernitz Biotechnology.
Below is the list of 10 most cited patents of Centivax:
| Publication Number | Citation Count |
| US11021531B1 | 7 |
| US11021532B1 | 4 |
| US10196427B2 | 4 |
| US11053304B1 | 3 |
| US11034762B1 | 3 |
| US11028167B1 | 3 |
| US9884893B2 | 3 |
| US20210060126A1 | 3 |
| US11028150B1 | 1 |
| US10836797B2 | 1 |
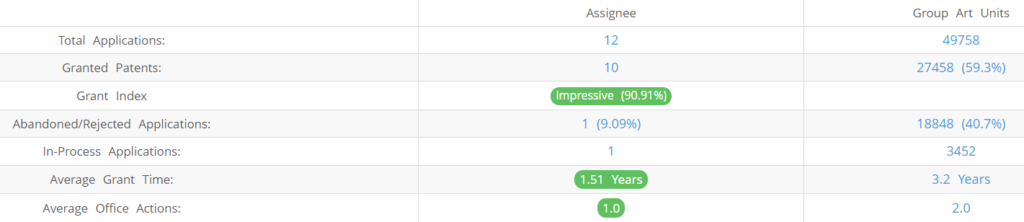
What Percentage of Centivax US Patent Applications Were Granted?
Centivax (Excluding its subsidiaries) has filed 12 patent applications at USPTO so far (Excluding Design and PCT applications). Out of these 10 have been granted leading to a grant rate of 90.91%.
Below are the key stats of Centivax patent prosecution at the USPTO.
Which Law Firms Filed Most US Patents for Centivax?
| Law Firm | Total Applications | Success Rate |
| Wilson Sonsini Goodrich & Rosati | 12 | 90.91% |
List of Centivax patents
| Centivax Patents | Title |
| US11560409B2 | Epitope Focusing By Variable Effective Antigen Surface Concentration |
| US11053304B1 | Anti-Sars-Cov-2 Antibodies Derived From 6Nb6 |
| US11034762B1 | Anti-Sars-Cov-2 Antibodies Derived From Cr3022 |
| US11028167B1 | Anti-Sars-Cov-2 Antibodies Derived From 3Bgf |
| US11028150B1 | Anti-Sars-Cov-2 Antibodies Derived From 2Dd8 |
| US11021532B1 | Superhuman Anti-Sars-Cov-2 Antibodies And Uses Thereof |
| US11021531B1 | Anti-Sars-Cov-2 Antibodies Derived From 2Ghw |
| US10836797B2 | Epitope Focusing By Variable Effective Antigen Surface Concentration |
| US10196427B2 | Epitope Focusing By Variable Effective Antigen Surface Concentration |
| US9884893B2 | Epitope Focusing By Variable Effective Antigen Surface Concentration |
| US20230312658A1 | Epitope Focusing By Variable Effective Antigen Surface Concentration |
| US20230272051A1 | Anti-Sars-Cov-2 Antibodies Derived From 2Dd8 |
| US20220088175A1 | Optimized Vaccine Compositions And Methods For Making The Same |
| US20210060126A1 | Major Histocompatibility Complex (Mhc) Compositions And Methods Of Use Thereof |
| EP3927372A4 | Optimized Vaccine Compositions And Methods For Making The Same |
| EP3727467A4 | Major Histocompatibility Complex (Mhc) Compositions And Methods Of Use Thereof |
| HK40066588A | Optimized Vaccine Compositions And Methods For Making The Same |
| HK40040284A | Major Histocompatibility Complex (Mhc) Compositions And Methods Of Use Thereof |
| APP2023015292A0 | Anti-Venom Antibodies And Uses Thereof |
| ZA202310422A | Anti-Venom Antibodies And Uses Thereof |
| AU2022254745A1 | Anti-Venom Antibodies And Uses Thereof |
| US20210147566A1 | Antibodies And Uses Thereof |
| EP3937179A1 | Epitope Focusing By Variable Effective Antigen Surface Concentration |
| WO2022217116A1 | Anti-Venom Antibodies And Uses Thereof |
| WO2021194891A1 | Anti-Sars-Cov-2 Antibodies Derived From Cr3022 |
| WO2021194896A1 | Anti-Sars-Cov-2 Antibodies Derived From 2Ghw |
| WO2021194985A1 | Anti-Sars-Cov-2 Antibodies Derived From 6Nb6 |
| WO2021194951A1 | Anti-Sars-Cov-2 Antibodies Derived From 2Dd8 |
| WO2021194965A1 | Superhuman Anti-Sars-Cov-2 Antibodies And Uses Thereof |
| WO2021194886A1 | Anti-Sars-Cov-2 Antibodies Derived From 3Bgf |
| WO2021076931A1 | Antibodies And Uses Thereof |
What are Centivax key innovation segments?
What Technologies are Covered by Centivax?

The chart below distributes patents filed by Centivax
