Dracen Pharmaceuticals has a total of 41 patents globally, out of which no patents have been granted. Of these 41 patents, more than 85% patents are active. Europe is where Dracen Pharmaceuticals has filed the maximum number of patents, followed by Japan, United States of America and Israel. Parallelly, United States of America seems to be the main focused R&D centre and also is the origin country of Dracen Pharmaceuticals.
Dracen Pharmaceuticals was founded in 2016. The company creates innovative glutamine antagonists in order to enhance the results of cancer patients.
Do read about some of the most popular patents of Dracen Pharmaceuticals which have been covered by us in this article and also you can find Dracen Pharmaceuticals patents information, the worldwide patent filing activity and its patent filing trend over the years, and many other stats over Dracen Pharmaceuticals patent portfolio.
How many patents does Dracen Pharmaceuticals have?
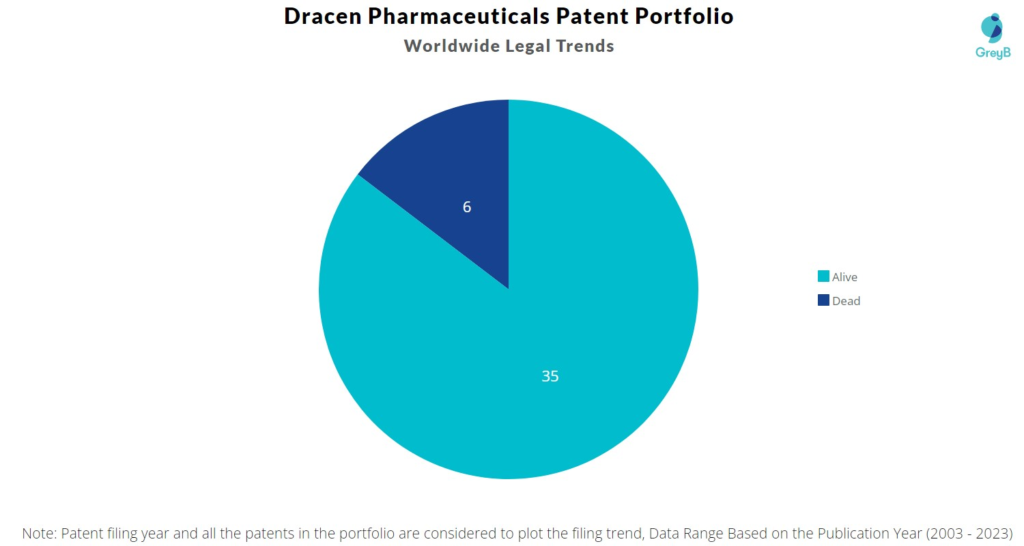
Dracen Pharmaceuticals has a total of 41 patents globally. These patents belong to 6 unique patent families. Out of 41 patents, 35 patents are active.
How Many Patents did Dracen Pharmaceuticals File Every Year?
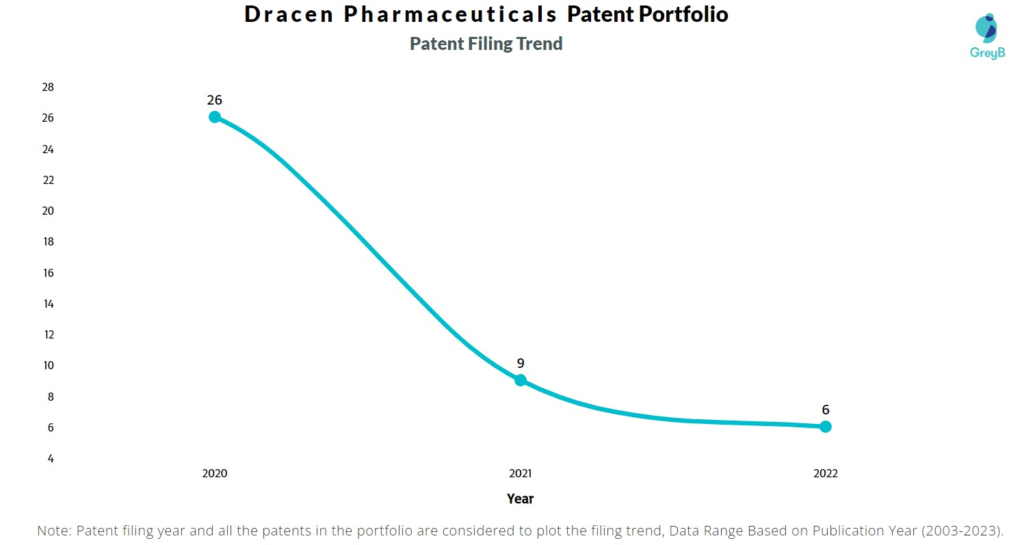
Are you wondering why there is a drop in patent filing for the last two years? It is because a patent application can take up to 18 months to get published. Certainly, it doesn’t suggest a decrease in the patent filing.
| Year of Patents Filing or Grant | Dracen Pharmaceuticals Applications Filed | Dracen Pharmaceuticals Patents Granted |
| 2023 | – | 1 |
| 2021 | 9 | – |
| 2020 | 26 | – |
| 2022 | 6 | – |
How many Dracen Pharmaceuticals patents are Alive/Dead?
Worldwide Patents
How Many Patents did Dracen Pharmaceuticals File in Different Countries?
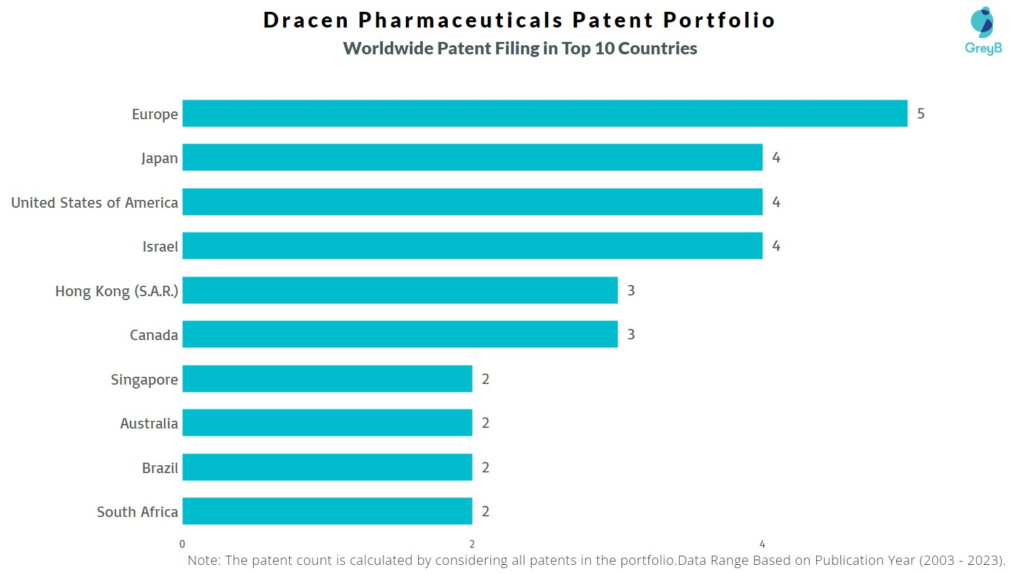
Countries in which Dracen Pharmaceuticals Filed Patents
| Country | Patent |
| Europe | 5 |
| Japan | 4 |
| United States of America | 4 |
| Israel | 4 |
| Hong Kong (S.A.R.) | 3 |
| Canada | 3 |
| Singapore | 2 |
| Australia | 2 |
| Brazil | 2 |
| South Africa | 2 |
| Mexico | 2 |
| India | 2 |
| New Zealand | 1 |
Where are Research Centers of Dracen Pharmaceuticals Patents Located?
The Research Center for Dracen Pharmaceuticals is the United States of America.
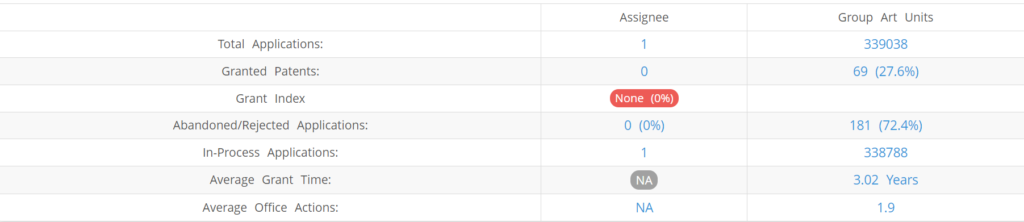
What Percentage of Dracen Pharmaceuticals US Patent Applications Were Granted?
Dracen Pharmaceuticals (Excluding its subsidiaries) has filed 1 patent applications at USPTO so far (Excluding Design and PCT applications). Out of these 0 have been granted leading to a grant rate of 0%.
Below are the key stats of Dracen Pharmaceuticals patent prosecution at the USPTO.
Which Law Firms Filed Most US Patents for Dracen Pharmaceuticals?
| Law Firm | Total Applications | Success Rate |
| Sterne Kessler Goldstein & Fox | 1 | 0% |
List of Dracen Pharmaceuticals patents
| Dracen Pharmaceuticals Patents | Title |
| US11760723B2 | Method Of Preparing A Don Prodrug From L-Pyroglutamic Acid |
| US20220332676A1 | Lyophilized Composition Comprising (S)-Isopropyl 2-((S)-2-Acetamido-3-(1H-Indol-3-Yl)Propanamido)-6-Diazo-5-Oxohexanoate For Intravenous Administration And The Use Thereof |
| US20220117938A1 | Combinaton Therapy With A Don Prodrug And An Immune Checkpoint Inhibitor |
| US20220089522A1 | Method Of Preparing A Don Prodrug From L-Glutamic Acid |
| EP4037674A4 | Lyophilized Composition Comprising (S)-Isopropyl 2-((S)-2-Acetamido-3-(1H-Indol-3-Yl)Propanamido)-6-Diazo-5-Oxohexanoate For Intravenous Administration And The Use Thereof |
| EP4221842A1 | Lyophilized Composition Comprising (S)-Isopropyl 2-((S)-2- Acetamido-3-(1H-Indol-3-Yl)Propanamido)-6-Diazo-5- Oxohexanoate For Subcutaneous Administration And The Use Thereof |
| EP3923934A4 | Method Of Preparing A Don Prodrug From L-Pyroglutamic Acid |
| EP3911310A4 | Combinaton Therapy With A Don Prodrug And An Immune Checkpoint Inhibitor |
| WO2022261117A1 | Combination Therapy With A Don Prodrug And A Tigit Inhibitor |
| CA3153424A1 | Lyophilized Composition Comprising (S)-Isopropyl 2-((S)-2-Acetamido-3-(1H-Indol-3-Yl)Propanamido)-6-Diazo-5-Oxohexanoate For Intravenous Administration And The Use Thereof |
| CA3129136A1 | Method Of Preparing A Don Prodrug From L-Pyroglutamic Acid |
| CA3126822A1 | Combinaton Therapy With A Don Prodrug And An Immune Checkpoint Inhibitor |
| JP2022518232A5 | Combination Therapy With Don Prodrugs And Immune Checkpoint Inhibitors |
| JP2022552168A | Frozen Containing (S)-Isopropyl 2-((S)-2-Acetamido-3-(1H-Indol-3-Yl)Propanamide)-6-Diazo-5-Oxohexanoate For Intravenous Administration Dry Composition And Its Use |
| JP2022519574A | Method For Preparing Don Prodrugs From L-Glutamic Acid |
| JP2022519530A | Method For Preparing Don Prodrugs From L-Pyroglutamic Acid |
| AU2020356925A1 | Lyophilized Composition Comprising (S)-Isopropyl 2-((S)-2-Acetamido-3-(1H-Indol-3-Yl)Propanamido)-6-Diazo-5-Oxohexanoate For Intravenous Administration And The Use Thereof |
| AU2020208637A1 | Combinaton Therapy With A Don Prodrug And An Immune Checkpoint Inhibitor |
| HK40066185A | Method Of Preparing A Don Prodrug From L-Glutamic Acid |
| HK40065802A | Method Of Preparing A Don Prodrug From L-Pyroglutamic Acid |
| HK40062578A | Combinaton Therapy With A Don Prodrug And An Immune Checkpoint Inhibitor |
| MX2022004014A | Lyophilized Composition Comprising (S)-Isopropyl 2-((S)-2-Acetamido-3-(1H-Indol-3-Yl)Propanamido)-6-Diazo-5-Oxoh Exanoate For Intravenous Administration And The Use Thereof. |
| MX2021008546A | Combinaton Therapy With A Don Prodrug And An Immune Checkpoint Inhibitor. |
| IL291850A | Lyophilized Composition Comprising (S)-Isopropyl 2-((S)-2-Acetamido-3-(1H-Indol-3-Yl)Propanamido)-6-Diazo-5-Oxohexanoate For Intravenous Administration And The Use Thereof |
| ZA202204864A | Lyophilized Composition Comprising (S)-Isopropyl 2-((S)-2-Acetamido-3-(1H-Indol-3-Yl)Propanamido)-6-Diazo-5-Oxohexanoate For Intravenous Administration And The Use Thereof |
| SG11202203349QA | Lyophilized Composition Comprising (S)-Isopropyl 2-((S)-2-Acetamido-3-(1H-Indol-3-Yl)Propanamido)-6-Diazo-5-Oxohexanoate For Intravenous Administration And The Use Thereof |
| IN202117039458A | Method Of Preparing A Don Prodrug From L-Glutamic Acid |
| IN202117037862A | Method Of Preparing A Don Prodrug From L-Pyroglutamic Acid |
| BR112021014112A2 | Uses Of Don Pro-Drug Compound And Immune Control Point Inhibitor To Treat Cancer, As Well As Compound And Pharmaceutical Composition Comprising The Same |
| IL285470A | Method Of Preparing A Don Prodrug From L-Glutamic Acid |
| IL285459A | Method Of Preparing A Don Prodrug From L-Pyroglutamic Acid |
| IL284907A | Combinaton Therapy With A Don Prodrug And An Immune Checkpoint Inhibitor |
| SG11202107317WA | Combinaton Therapy With A Don Prodrug And An Immune Checkpoint Inhibitor |
| ZA202105841A | Combinaton Therapy With A Don Prodrug And An Immune Checkpoint Inhibitor |
| NZ787820A | Lyophilized Composition Comprising (S)-Isopropyl 2-((S)-2-Acetamido-3-(1H-Indol-3-Yl)Propanamido)-6-Diazo-5-Oxohexanoate For Intravenous Administration And The Use Thereof |
| EP3924330A4 | Method Of Preparing A Don Prodrug From L-Glutamic Acid |
| WO2022072820A1 | Lyophilized Composition Comprising (S)-Isopropyl 2-((S)-2- Acetamido-3-(1H-Indol-3-Yl)Propanamido)-6-Diazo-5- Oxohexanoate For Subcutaneous Administration And The Use Thereof |
| WO2020167829A1 | Method Of Preparing A Don Prodrug From L-Pyroglutamic Acid |
| WO2020167831A1 | Method Of Preparing A Don Prodrug From L-Glutamic Acid |
| WO2020150639A1 | Combinaton Therapy With A Don Prodrug And An Immune Checkpoint Inhibitor |
| BR112022006409A2 | Lyophylized Composition Comprising (S)-Isopropyl 2-((S)-2-Acetamido-3-(1H-Indol-3-Yl)Propanamido)-6-Diazo-5-Oxo-Hexanoate For Intravenous Administration And Use Of Same |
What are Dracen Pharmaceuticals key innovation segments?
What Technologies are Covered by Dracen Pharmaceuticals?

The chart below distributes patents filed by Dracen Pharmaceuticals
