Lipella Pharmaceuticals has a total of 34 patents globally, out of which 5 have been granted. Of these 34 patents, More than 20% patents are active. The United States of America is where Lipella Pharmaceuticals has filed the maximum number of patents, followed by Europe and Japan. Parallelly United States of America seems to be the main focused R&D center and also is the origin country of Lipella Pharmaceuticals.
Lipella Pharmaceuticals was founded in 2005 by Michael Chancellor and Jonathan Kaufman. Lipella Pharmaceuticals operates as a clinical-stage biotechnology company. The Company focuses on developing proprietary drug delivery platform that optimizes drug delivery to mucosal membranes for the treatment of cancer survivors. Lipella Pharmaceuticals Pharmaceuticals serves customers in the United States.
Do read about some of the most popular patents of Lipella Pharmaceuticals which have been covered by us in this article and also you can find Lipella Pharmaceuticals patents information, the worldwide patent filing activity and its patent filing trend over the years, and many other stats over Lipella Pharmaceuticals patent portfolio.
How many patents does the Founder and CEO of Lipella Pharmaceuticals have?
The Founders and CEO Jonathan Kaufman has 29 patents and Co-Founder Michael Chancellor has 163 Patents.
How many patents does Lipella Pharmaceuticals have?
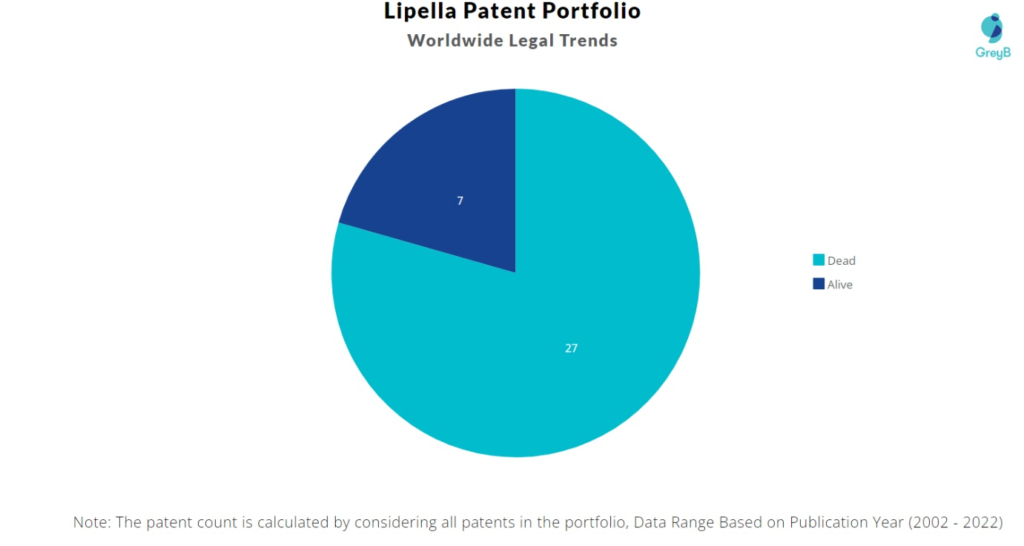
Lipella Pharmaceuticals has a total of 34 patents globally. These patents belong to 6 unique patent families. Out of 34 patents, 7 patents are active.
How Many Patents did Lipella Pharmaceuticals File Every Year?
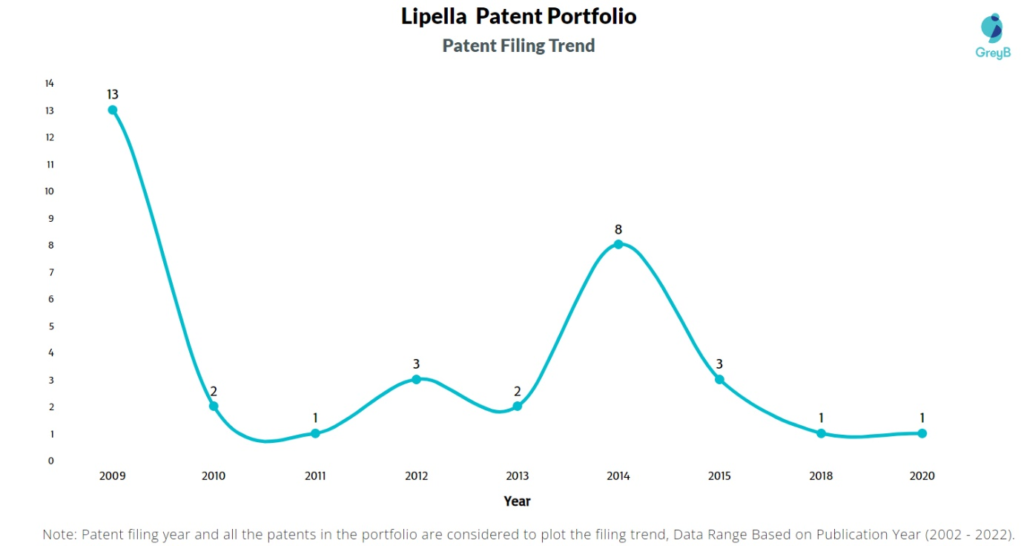
Are you wondering why there is a drop in patent filing for the last two years? It is because a patent application can take up to 18 months to get published. Certainly, it doesn’t suggest a decrease in the patent filing.
| Year of Patents Filing or Grant | Lipella Pharmaceuticals Applications Filed | Lipella Pharmaceuticals Patents Granted |
| 2009 | 13 | – |
| 2010 | 2 | – |
| 2011 | 1 | – |
| 2012 | 3 | 1 |
| 2013 | 2 | – |
| 2014 | 8 | 1 |
| 2015 | 3 | – |
| 2018 | 1 | – |
| 2020 | 1 | 2 |
| 2022 | – | 1 |
How many Lipella Pharmaceuticals patents are Alive/Dead?
Worldwide Patents
How Many Patents did Lipella Pharmaceuticals File in Different Countries?
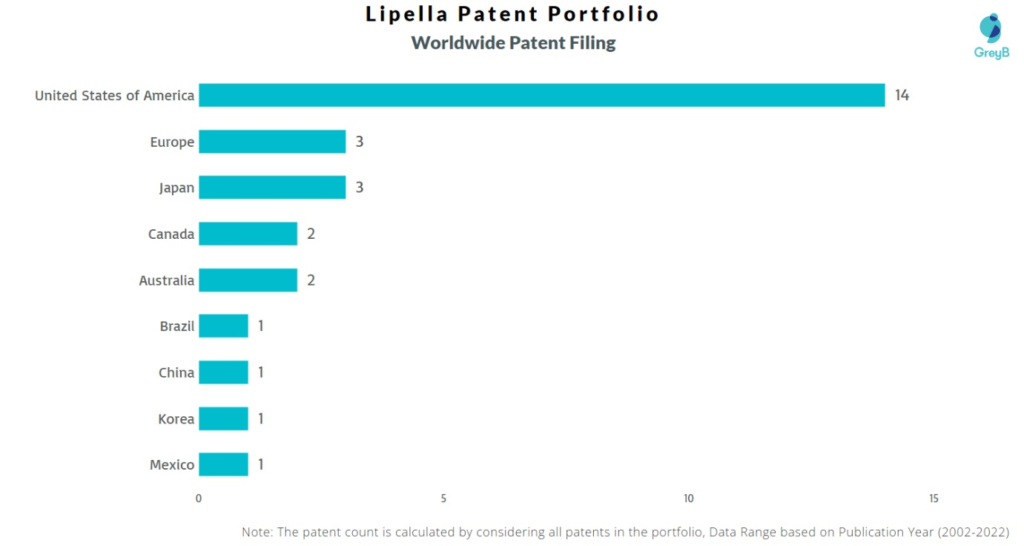
Countries in which Lipella Pharmaceuticals Filed Patents
| Country | Patents |
| United States of America | 14 |
| Europe | 3 |
| Japan | 3 |
| Canada | 2 |
| Australia | 2 |
| Brazil | 1 |
| China | 1 |
| Korea | 1 |
| Mexico | 1 |
Where are Research Centers of Lipella Pharmaceuticals Patents Located?
The research centers of all the Lipella Pharmaceuticals patents is the United States of America.
10 Best Lipella Pharmaceuticals Patents
US20100166739A1 is the most popular patent in the Lipella Pharmaceuticals portfolio. It has received 28 citations so far from companies like Boston Scientific, Medtronic and Mologic Limited.
Below is the list of 10 most cited patents of Lipella Pharmaceuticals:
| Publication Number | Citation Count |
| US20100166739A1 | 28 |
| US20130071466A1 | 26 |
| EP2273976A2 | 21 |
| US20120263781A1 | 12 |
| WO2009139984A2 | 9 |
| US20100104631A1 | 5 |
| US20150030667A1 | 4 |
| US20150037402A1 | 3 |
| WO2015154007A1 | 3 |
| US20160045623A1 | 2 |
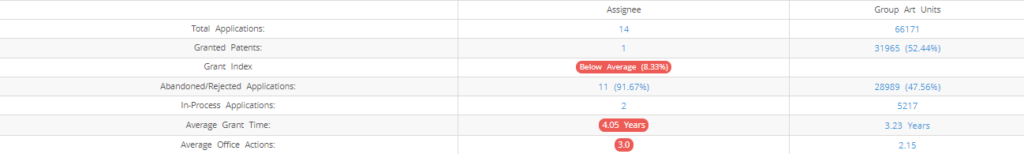
What Percentage of Lipella Pharmaceuticals US Patent Applications were Granted?
Lipella Pharmaceuticals (Excluding its subsidiaries) has filed 14 patent applications at USPTO so far (Excluding Design and PCT applications). Out of these 1 have been granted leading to a grant rate of 8.33%.
Below are the key stats of Lipella Pharmaceuticals patent prosecution at the USPTO.
Which Law Firms Filed Most US Patents for Lipella Pharmaceuticals
| Law Firm | Total Applications | Success Rate |
| Pabst Patent Group Llp | 10 | 11.11% |
| Troutman Pepper Hamilton Sanders Llp | 4 | 0.00% |
List of Lipella Pharmaceuticals Patents –
| Publication Number | Title |
| US11357725B2 | Delivery Of Agents Using Metastable Liposomes |
| US10639278B2 | Delivery Of Agents Using Metastable Liposomes |
| US20190094323A1 | Systems And Methods Of Detecting Interstitial Cystitis |
| US20170189564A9 | Systems And Methods Of Detecting Interstitial Cystitis |
| US20160045623A1 | Systems And Methods To Image Intercellular And Intercompartmental Defects With Magnetic Resonance Imaging (Mri) |
| US20150037402A1 | Methods And Compositions For Treating Gastric Disorders |
| US20150030667A1 | Compositions And Methods For Treating Underactive Bladder |
| US20130071466A1 | Methods And Compositions For Treating Gastric Disorders |
| US20120301540A1 | Method Of Treatment For Bladder Dysfunction |
| US20120263781A1 | Methods And Compositions For Treating Rhinitis |
| US20120093920A1 | Method Of Treatment For Bladder Dysfunction |
| US20110274745A1 | Instillation Of Liposomal Formulation Of Sirna And Antisense Oligonucleotides |
| US20100166739A1 | Methods And Compositions For Diagnosing Urological Disorders |
| US20100104631A1 | Method Of Treatment For Bladder Dysfunction |
| EP3060197A1 | Delivery Of Agents Using Metastable Liposomes |
| EP2599476A1 | Treatment Of Bladder Dysfunction Using Liposomal Botulinum Toxin |
| EP2273976A2 | Treatment Of Bladder Dysfunction Using Liposomal Botulinum Toxin |
| CN102065841A | Treatment Of Bladder Dysfunction Using Liposomal Botulinum Toxin |
| WO2015061449A9 | Delivery Of Agents Using Metastable Liposomes |
| WO2015154007A1 | Systems And Methods Of Detecting Interstitial Cystitis |
| WO2013116822A1 | Methods And Compositions For Treating Gastric Disorders |
| WO2011060065A3 | Instillation Of Liposomal Formulation Of Sirna And Antisense Oligonucleotides |
| WO2010078403A3 | Methods And Compositions For Diagnosing Urological Disorders |
| WO2009139984A2 | Method Of Treatment For Bladder Dysfunction |
| JP2016534087A | Drug Delivery Using Metastable Liposomes |
| JP5538359B2 | Treatment Of Bladder Dysfunction Using Liposomal Botulinum Toxin |
| JP2014062125A | Treatment For Bladder Dysfunction Using Liposomal Botulinum Toxin |
| AU2014340137B2 | Delivery Of Agents Using Metastable Liposomes |
| CA2927356A1 | Delivery Of Agents Using Metastable Liposomes |
| AU2009246834B2 | Treatment Of Bladder Dysfunction Using Liposomal Botulinum Toxin |
| CA2720523A1 | Treatment Of Bladder Dysfunction Using Liposomal Botulinum Toxin |
| BRPI0911098A2 | Liposomal Formulation And Method For Treatment Of Hyperactive Bladder. |
| MX2010010635A | Treatment Of Bladder Dysfunction Using Liposomal Botulinum Toxin. |
| KR1020100131471A | Treatment Of Bladder Dysfunction Using Liposomal Botulinum Toxin |
EXCLUSIVE INSIGHTS COMING SOON!
