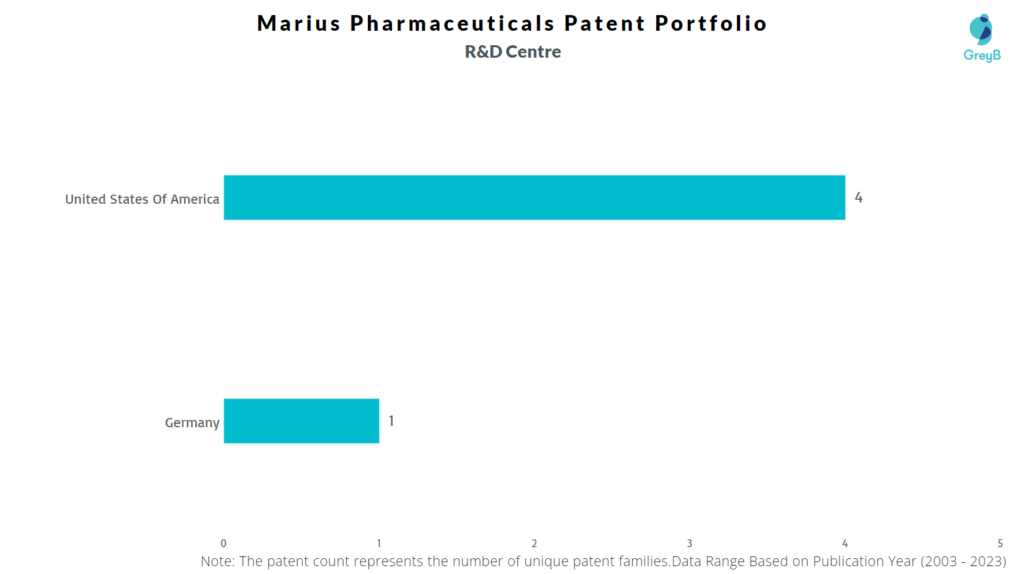
Marius Pharmaceuticals has a total of 30 patents globally, out of which 16 have been granted. Of these 30 patents, more than 76% patents are active. The United States is where Marius Pharmaceuticals has filed the maximum number of patents, followed by Europe (EPO). Parallelly, the USA seems to be the main focused R&D centre of Marius Pharmaceuticals and is also the origin country of the firm.
Marius Pharmaceuticals was founded in the year 2017 by Himanshu Shah and Om Dhingra. The company is a biopharmaceutical firm that brings novel treatments to a range of diseases with unmet medical needs.
Do read about some of the most popular patents of Marius Pharmaceuticals which have been covered by us in this article and also you can find Marius Pharmaceuticals patents information, the worldwide patent filing activity and its patent filing trend over the years, and many other stats over Marius Pharmaceuticals patent portfolio.
How many patents does Marius Pharmaceuticals have?
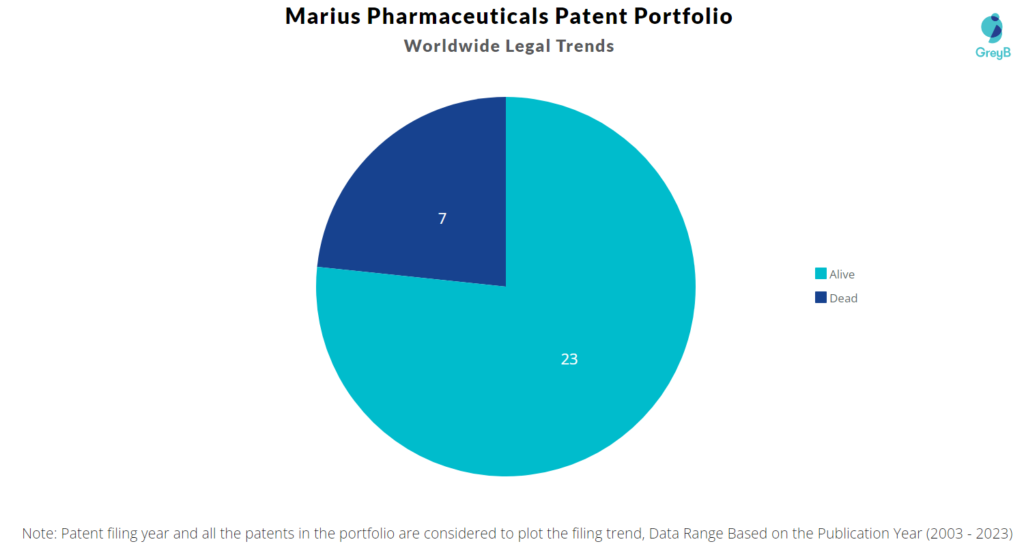
Marius Pharmaceuticals has a total of 30 patents globally. These patents belong to 5 unique patent families. Out of 30 patents, 23 patents are active.
How Many Patents did Marius Pharmaceuticals File Every Year?
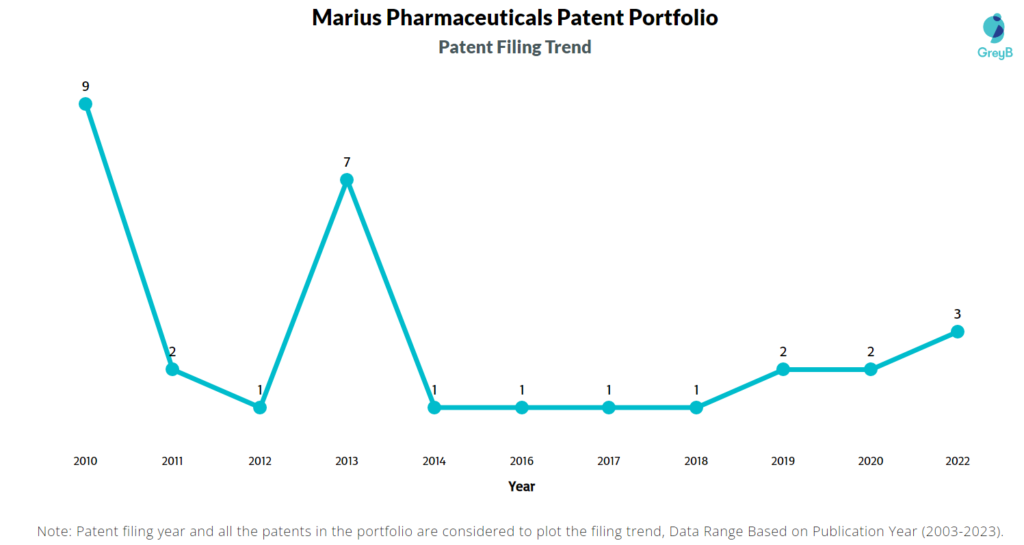
Are you wondering why there is a drop in patent filing for the last two years? It is because a patent application can take up to 18 months to get published. Certainly, it doesn’t suggest a decrease in the patent filing.
| Year of Patents Filing or Grant | Marius Pharmaceuticals Applications Filed | Marius Pharmaceuticals Patents Granted |
| 2022 | 3 | 1 |
| 2021 | – | 3 |
| 2020 | 2 | 2 |
| 2019 | 2 | 4 |
| 2018 | 1 | 3 |
| 2017 | 1 | 1 |
| 2016 | 1 | – |
| 2015 | – | – |
| 2014 | 1 | – |
| 2013 | 7 | – |
| 2012 | 1 | – |
| 2011 | 2 | – |
How many Marius Pharmaceuticals patents are Alive/Dead?
Worldwide Patents
How Many Patents did Marius Pharmaceuticals File in Different Countries?
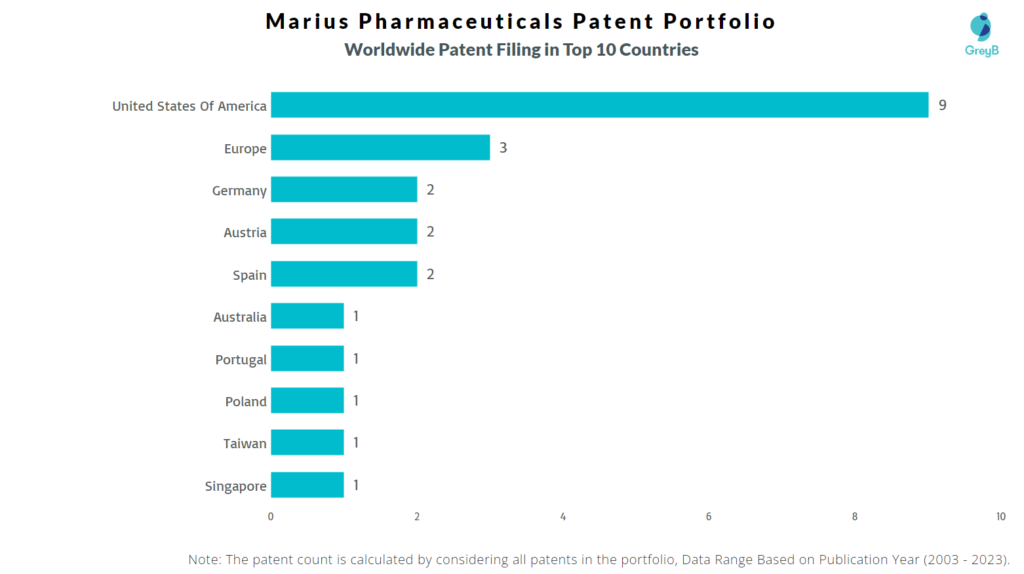
Countries in which Marius Pharmaceuticals Filed Patents
| Country | Patents |
| United States Of America | 9 |
| Europe | 3 |
| Germany | 2 |
| Austria | 2 |
| Spain | 2 |
| Australia | 1 |
| Portugal | 1 |
| Poland | 1 |
| Taiwan | 1 |
| Singapore | 1 |
| Canada | 1 |
| Hong Kong (S.A.R.) | 1 |
| Turkey | 1 |
| India | 1 |
| Macao | 1 |
| New Zealand | 1 |
Where are Research Centres of Marius Pharmaceuticals Patents Located?
Most Cited Marius Pharmaceuticals Patent
US20130303495A1 is the most popular patent in the Marius Pharmaceuticals portfolio. It has received 56 citations so far from companies like Merck Sharp & Dohme and Clarus Therapeutics.
List of Marius Pharmaceuticals
| Marius Pharmaceuticals Patents | Title |
| US11617758B2 | Emulsion Formulations |
| US11590146B2 | Modulation Of Solubility, Stability, Absorption, Metabolism, And Pharmacokinetic Profile Of Lipophilic Drugs By Sterols |
| US10576089B2 | Modulation Of Solubility, Stability, Absorption, Metabolism, And Pharmacokinetic Profile Of Lipophilic Drugs By Sterols |
| US10576090B2 | Modulation Of Solubility, Stability, Absorption, Metabolism, And Pharmacokinetic Profile Of Lipophilic Drugs By Sterols |
| US20220265678A1 | Preferred Oral Testosterone Undecanoate Therapy To Achieve Testosterone Replacement Treatment |
| EP2968137B1 | Emulsion Formulations |
| EP2519230B1 | Modulation Of Solubility, Stability, Absorption, Metabolism, And Pharmacokinetic Profile Of Lipophilic Drugs By Sterols |
| WO2022245933A8 | Preferred Oral Testosterone Undecanoate Therapy To Achieve Testosterone Replacement Treatment |
| ES2907284T3 | Emulsion Formulations |
| DE602013080022T2 | Emulsion Formulations |
| PL2519230T3 | Modulation Of Solubility, Stability, Absorption, Metabolism, And Pharmacokinetic Profile Of Lipophilic Drugs By Sterols |
| ES2710149T3 | Modulation Of Solubility, Stability, Absorption, Metabolism, And Pharmacokinetic Profile Of Lipophilic Drugs By Sterols |
| DE602010054305T2 | Modulation Of The Solution, Stability, Absorption, Metabolism And Pharmacokinetic Profile Of Lipophilic Active Substances Using Sterols |
| TR201900369T4 | Modulation Of Solubility, Stability, Absorption, Metabolism And Pharmacokinetic Profile Of Lipophilic Drugs By Sterols. |
| PT2519230T | Modulation Of Solubility, Stability, Absorption, Metabolism, And Pharmacokinetic Profile Of Lipophilic Drugs By Sterols |
| AU2022277557A1 | Preferred Oral Testosterone Undecanoate Therapy To Achieve Testosterone Replacement Treatment |
| CA2822435C | Modulation Of Solubility, Stability, Absorption, Metabolism, And Pharmacokinetic Profile Of Lipophilic Drugs By Sterols |
| HK1219061A1 | Emulsion Formulations |
| IN359823B | Modulation Of Solubility, Stability, Absorption, Metabolism, And Pharmacokinetic Profile Of Lipophilic Drugs By Sterols |
| TWI673068B | Emulsion Formulations |
| MOJ003534C | Emulsion Formulations. |
| NZ631833B | Emulsion Formulations |
| SG11202304706UA | Preferred Oral Testosterone Undecanoate Therapy To Achieve Testosterone Replacement Treatment |
| US20180021349A1 | Emulsion Formulations |
| US20130303495A1 | Emulsion Formulations |
| US20110263552A1 | Modulation Of Side Effect Profile Of 5-Alpha Reductase Inhibitor Therapy |
| US20110160168A1 | Modulation Of Solubility, Stability, Absorption, Metabolism, And Pharmacokinetic Profile Of Lipophilic Drugs By Sterols |
| EP2682111A1 | Modulation Of Solubility, Stability, Absorption, Metabolism, And Pharmacokinetic Profile Of Lipophilic Drugs By Sterols |
| AT1445416T | Emulsionsformulierungen |
| AT1050418T | Modulation Der Löslichkeit, Der Stabilität, Der Absorption, Des Stoffwechsels Und Des Pharmakokinetischen Profils Lipophiler Wirkstoffe Mithilfe Von Sterolen |
What are Marius Pharmaceuticals’ key innovation segments?
What Technologies are Covered by Marius Pharmaceuticals?

The chart below distributes patents filed by Marius Pharmaceuticals
