Treating cancer with fewer side effects and cutting down recovery time has always been the goal. And advancements in drug delivery, especially with nanotechnology have already achieved 30% more effectiveness. Now, nanorobotics is set to take this further.
Nanorobots are tiny machines, much smaller than human cells, designed to perform specific tasks inside the body. They are made from materials that are safe for the body, and their main job is to deliver medicine directly to where it’s needed.
These nanorobots move through the bloodstream, using special sensors to identify diseased cells. They are equipped with such technologies that they know when and where to release the medicine.
In some cases, doctors can even guide them using external tools like magnets or light. After finishing their task, nanorobots either naturally degrade or are safely removed from the body.
Patents Related to Nanorobotics
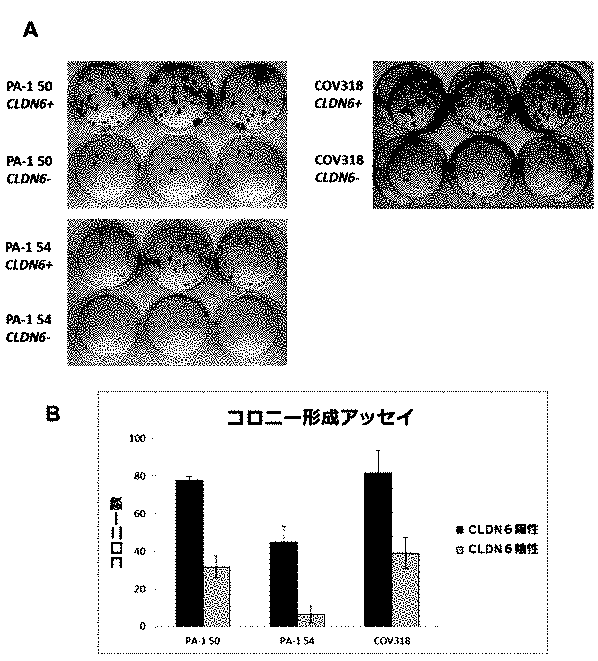
- US11337922B2: This patent focuses on CLDN6, a protein associated with cancer stem cells (CSCs), as a promising target for enhancing cancer treatment. Traditional therapies often struggle to eliminate CSCs, leading to treatment resistance and cancer recurrence. By targeting CLDN6, this patent proposes methods to detect and measure CSC levels, monitor therapy effectiveness, and treat cancer using CLDN6-targeting antibodies combined with chemotherapy. The goal is to inhibit or eliminate CSCs, improving the long-term success of cancer treatments and reducing relapse rates.
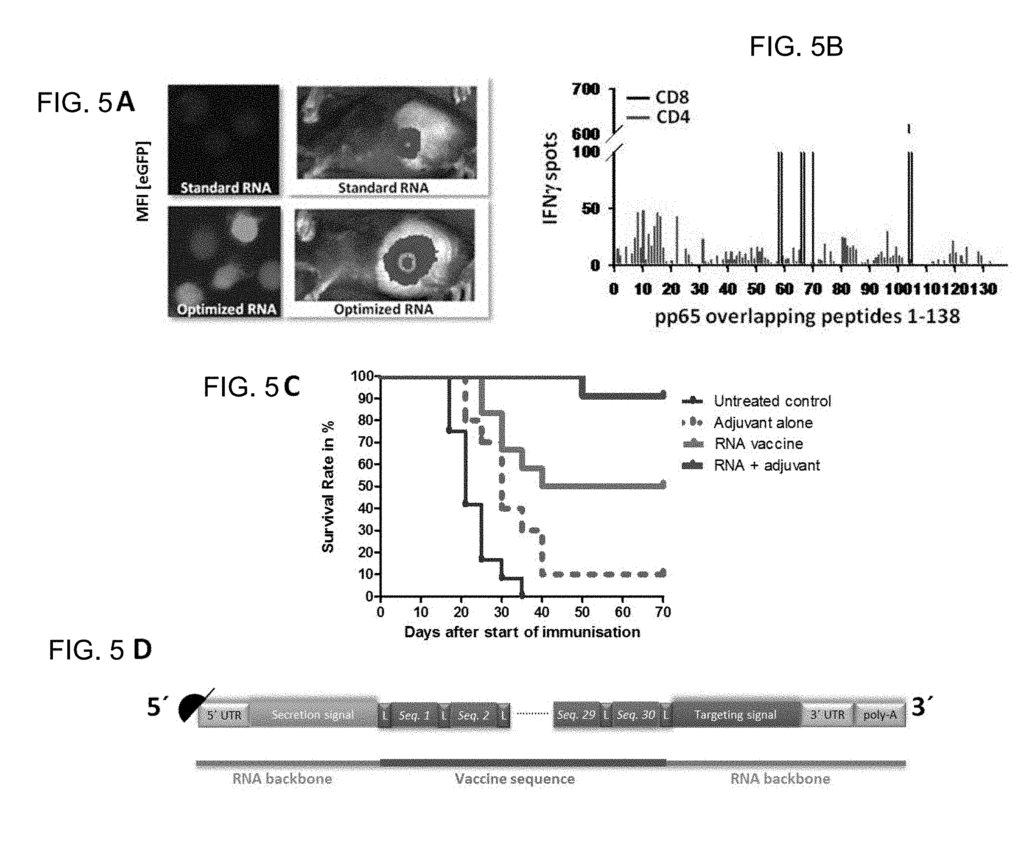
- US2019083593A1: This patent introduces a patient-specific cancer treatment that targets unique tumor antigen expression and individual tumor mutations. The invention outlines a two-step method: (i) inducing a first immune response against common tumor antigens and (ii) inducing a second immune response that is specific to the patient’s cancer mutations. By tailoring the immune responses to both shared tumor antigens and the individual’s unique cancer cell mutations, this approach aims to enhance the precision and effectiveness of cancer therapies, offering a more personalized and targeted treatment strategy for cancer patients.
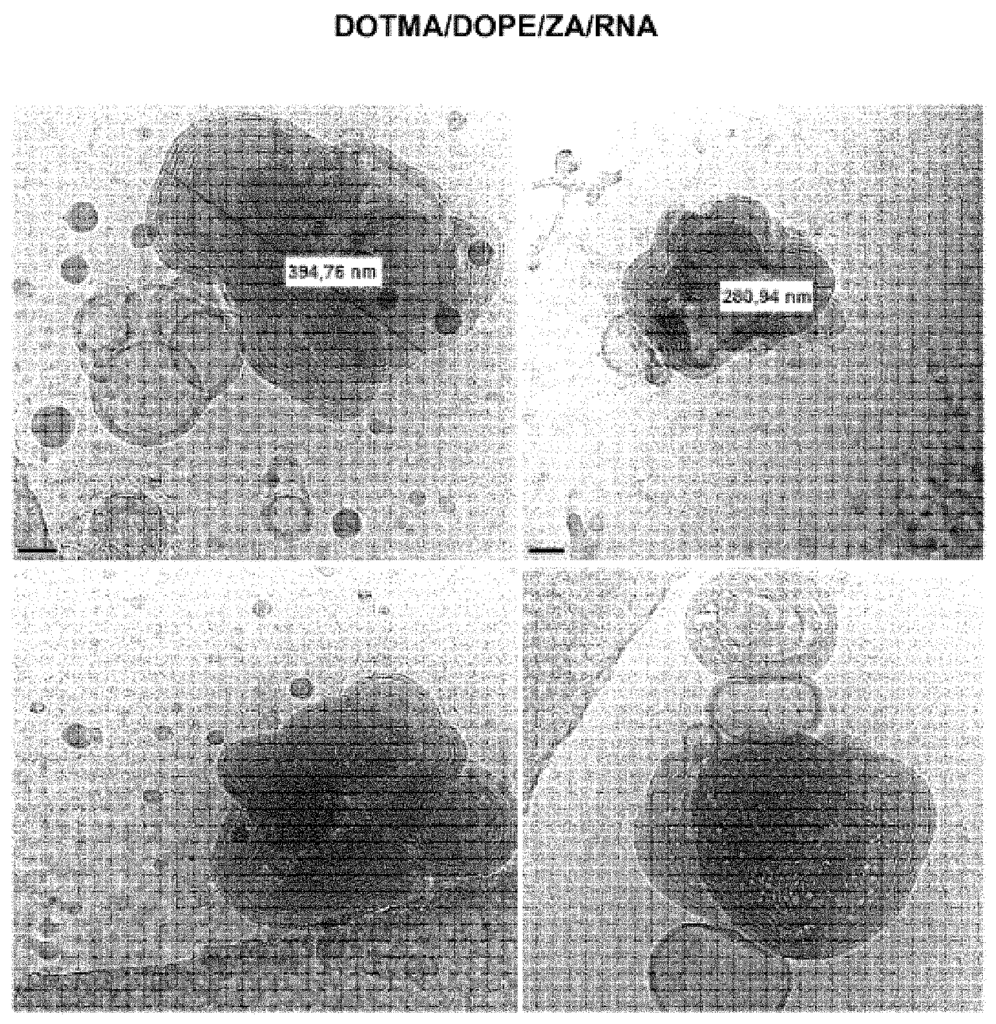
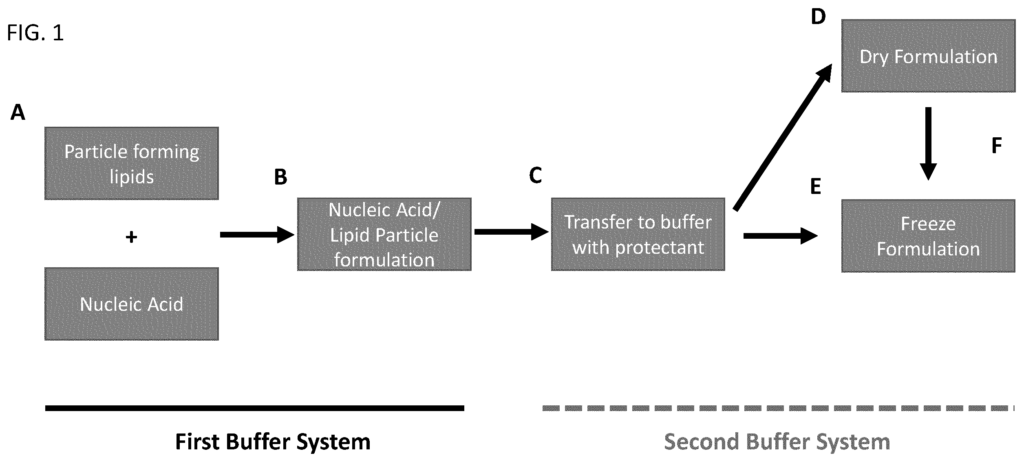
- US2024041785A1: This patent focuses on Lipid Nanoparticle (LNP) formulations designed to enhance the stability of mRNA vaccines at higher temperatures, such as refrigeration or room temperature. These formulations contain specific lipid compositions, including (4-hydroxy butyl) azanediyl bis(hexane-6,1-diyl) bis(2-hexyl decanoate), polyethylene glycol-2000, distearoylphosphatidylcholine, and cholesterol in defined ratios. Stabilizing agents like sucrose, trehalose, and Tris buffer are included to maintain the vaccine’s integrity. The combination of these lipids and stabilizers helps preserve the formulation, allowing for better temperature resilience and extended shelf life of mRNA vaccines.
- JP6799101B2: This patent focuses on Claudins (CLDN), which are membrane proteins critical for maintaining tight junctions in epithelial and endothelial cells. Specifically, Claudin 18 (CLDN18) has splice variants that are commonly expressed in cancer cells. The patent introduces bispecific antibodies that target both CLDN18 on tumor cells and CD3 on T cells, effectively activating the immune system to attack cancer cells. By binding to CLDN18 on tumor cells and engaging CD3 on T cells, these antibodies can induce T cell-mediated lysis of cancer cells, offering a promising approach to cancer immunotherapy.
- JP6865778B2: This patent focuses on CLDN6, a protein linked to cancer stem cells (CSCs), which are resistant to traditional cancer treatments and contribute to recurrence and metastasis. By targeting CLDN6, the invention enables more precise cancer therapy through detecting CLDN6-expressing cells, tracking CSC response to treatment, and using CLDN6-specific antibodies combined with chemotherapy. The goal is to inhibit or eliminate CSCs, improving the effectiveness and long-term outcomes of cancer treatments.
Challenges in Nanorobot Drug Delivery
1. Regulatory Hurdles
Bringing nanorobots from the lab to the market requires strict regulatory approvals. Agencies like the FDA need extensive safety testing before they can allow these technologies in treatments. Given the novelty of nanorobots, ensuring they meet safety standards for human use can take time, delaying their availability.
2. Manufacturing Complexities
Producing nanorobots at a large scale is tricky due to their microscopic size and intricate design. Ensuring that each nanorobots functions correctly without flaws adds to the complexity, making it difficult to manufacture them efficiently and cost-effectively.
3. Cost and Accessibility
The cost of developing and producing nanorobots is high, which could make treatments expensive initially. This could limit their availability to only a few patients, raising concerns about affordability in the broader healthcare system.
4. Biocompatibility and Safety Concerns
Since nanorobots interact with the body at a cellular level, there are risks of side effects like immune reactions or toxicity. Ensuring that nanorobots are safe to use and don’t cause long-term health issues is a critical challenge that needs careful research and testing.
Conclusion
As nanorobotics advances, it will revolutionize medicine in ways beyond today’s capabilities. Companies like Deepcell, Bionaut Labs, and IBM are developing nano-scale solutions for diagnostics, drug delivery, and cellular manipulation. What sets nanorobots apart is their precision, programmability, and ability to interact directly with cells. Unlike other drug delivery systems, nanorobots can navigate the body, perform specific tasks, and provide real-time feedback while minimizing side effects.
Nanorobots have the potential to address major medical challenges, from improving cancer treatment to aiding tissue regeneration and repairing genetic defects. As research progresses and production scales, nanorobots will usher in an era of personalized medicine, where treatments are not only more effective but also tailored to individuals. This marks a major leap toward the future of healthcare.