For more than two decades, one class of drugs has quietly transformed the treatment of autoimmune diseases. Therapies that block TNF-α-a key driver of inflammation-have given patients with rheumatoid arthritis, Crohn’s disease, and psoriasis a level of disease control that was once unimaginable. But that success has come with a structural compromise: these therapies almost always require injections.
Forward therapeutics patent US12521368B2 revisits a long-standing ambition in immunology-treating TNF-α-driven disease with oral medication. Rather than introducing a finished drug, the patent stakes out intellectual territory around small-molecule compounds designed to modulate TNF-α activity. In doing so, it reflects a broader industry effort to rethink whether one of medicine’s most valuable targets can finally move beyond the needle.
Why TNF-α Therapies Became Injection-Only
TNF-α is a powerful immune signaling protein. When tightly regulated, it helps the body respond to infection. When overproduced, it drives chronic inflammation and tissue damage. Blocking TNF-α has therefore become one of the most validated strategies in autoimmune disease.
The challenge lies in the protein itself. TNF-α is large, dynamic, and functions through complex protein–protein interactions. These characteristics make it an ideal target for biologics-large antibody-based drugs-but a frustrating one for traditional pills.
Small-molecule drugs work best when they can latch onto a well-defined pocket on a target protein. TNF-α offers few such footholds. As a result, oral TNF-α inhibitors have repeatedly failed in development, while injectable biologics became the industry standard despite their cost, inconvenience, and systemic immune effects.
Problem and Solution: Reopening a Closed Drug Target
The problem is not that TNF-α is unimportant-it is that it has resisted conventional small-molecule drug design. The industry largely accepted injections as the unavoidable price of efficacy.
The approach outlined in this patent is to challenge that assumption at the chemistry level. Instead of claiming a single drug candidate, the patent defines a broad family of small molecules designed to interfere with TNF-α signaling. The emphasis is not on a final therapeutic product, but on establishing a chemical framework that could support oral modulation of a historically difficult target.
This represents a strategic shift from “find the perfect molecule” to “secure the chemical design space early.”
Keep track of every patent move and monitor competitor activity in real-time. Click here for full insights:
How the Patent’s Strategy Works
Rather than focusing narrowly on direct TNF-α binding, the patent claims a scaffold-a shared molecular backbone-that can be modified in many ways. These variations are designed to explore different modes of interaction with TNF-α or it’s signaling pathways.
By covering a wide range of related compounds, the patent creates flexibility. Some variants may prove ineffective. Others may show partial activity, improved absorption, or acceptable safety profiles. The value lies in protecting the entire exploration process, not just a single result.
In practical terms, this kind of patent functions as a foundation for future optimization, rather than a declaration of clinical success.
What the Patent Explicitly Covers
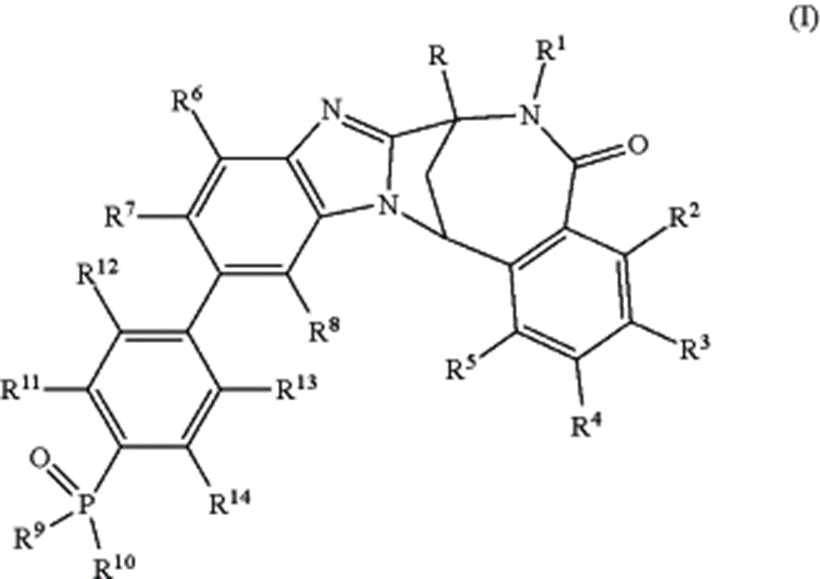
The image depicts the broadly defined chemical scaffold claimed in the patent, with multiple substitution positions (R, R¹–R¹⁴). These variable positions define the scope of the claimed chemical genus and allow extensive medicinal chemistry flexibility. Importantly, this structure represents intellectual property scope, not a binding pose, active conformation, or mechanistic model.
The patent claims a broad chemical genus (Formula I) encompassing numerous substituted variants, including:
- non‑deuterated and deuterated compounds,
- pharmaceutically acceptable salts and solvates,
- N‑oxide forms, and
- pharmaceutical compositions containing these compounds.
The title and abstract clearly state that these compounds function as TNF‑α inhibitors and are intended for use in the treatment of diseases or disorders associated with TNF‑α activity. At a minimum, the patent establishes composition‑of‑matter protection around a family of small molecules positioned as TNF‑α modulators.
Strategic and Competitive Implications
From a business perspective, the appeal is obvious. TNF-α biologics represent one of the largest drug markets in the world. Even a moderately effective oral alternative could reshape treatment economics, patient adherence, and healthcare delivery.
At the same time, the patent reflects the reality that oral TNF-α modulation remains uncertain. Many past attempts failed late in development. This patent does not resolve those risks-but it ensures that if chemistry finally catches up to ambition, the underlying intellectual property is already secured.
More broadly, the filing illustrates a renewed willingness in pharma to revisit “closed” targets using new chemical thinking, rather than accepting biological limitations as final.
From Proven Biology to New Delivery Paradigms
Patent US12521368B2 does not announce the end of injectable TNF-α therapies. Instead, it marks a re-entry point into a problem the industry once set aside. By claiming a versatile small-molecule design space, the invention keeps open the possibility that oral modulation of TNF-α could one day become practical.
The long-term significance lies in what this represents: a shift from accepting delivery constraints to questioning them. As chemistry, computation, and screening tools evolve, targets that once seemed incompatible with pills may no longer remain so. Whether this particular approach succeeds remains to be seen-but the patent ensures that if the needle can be replaced, its path will be legally and strategically defined.
Want to monitor patent activity around oral alternatives to injectable biologics? Fill out the form to receive a customized patent insight brief on biopharma IP trends, chemical scaffolds, and therapeutic delivery innovation.


