Fzata has a total of 13 patents globally, out of which no patents have been granted. Of these 13 patents, more than 76% patents are active. Canada is where Fzata has filed the maximum number of patents, followed by the United States of America and Europe. Parallelly, United States of America seems to be the main focused R&D centre and also is the origin country of Fzata.
Fzata was founded in 2015. Fzata has a new way of making medicines that you take by mouth, and it’s called Bioengineered Probiotic Yeast Medicines (BioPYM). They have more than 10 drug options for treating conditions in the digestive system.
Do read about some of the most popular patents of Fzata which have been covered by us in this article and also you can find Fzata patents information, the worldwide patent filing activity and its patent filing trend over the years, and many other stats over Fzata patent portfolio.
How many patents does Fzata have?
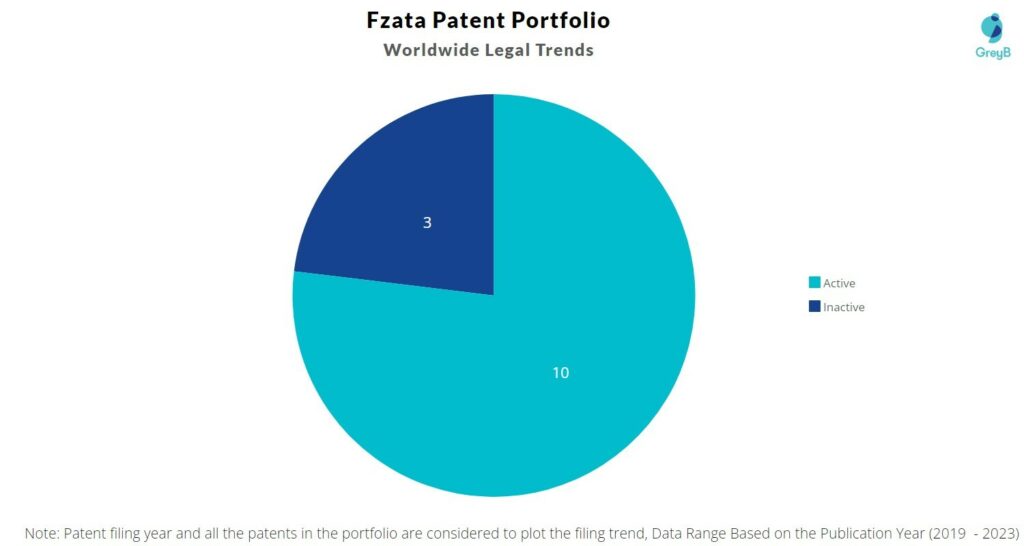
Fzata has a total of 13 patents globally. These patents belong to 3 unique patent families. Out of 13 patents, 10 patents are active.
How Many Patents did Fzata File Every Year?
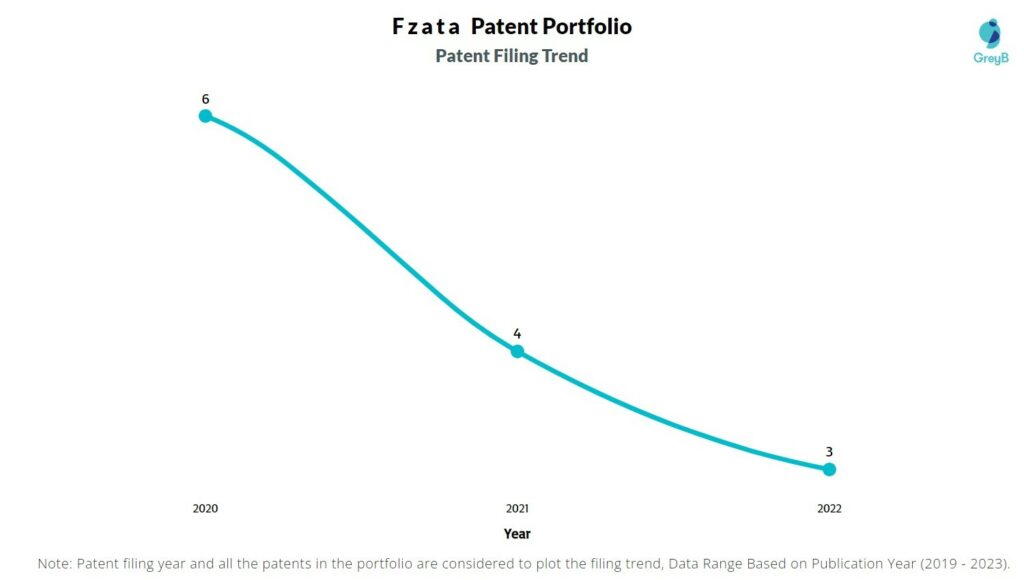
Are you wondering why there is a drop in patent filing for the last two years? It is because a patent application can take up to 18 months to get published. Certainly, it doesn’t suggest a decrease in the patent filing.
| Year of Patents Filing or Grant | Fzata Applications Filed | Fzata Patents Granted |
| 2022 | 3 | – |
| 2021 | 4 | – |
| 2020 | 6 | – |
How many Fzata patents are Alive/Dead?
Worldwide Patents
How Many Patents did Fzata File in Different Countries?
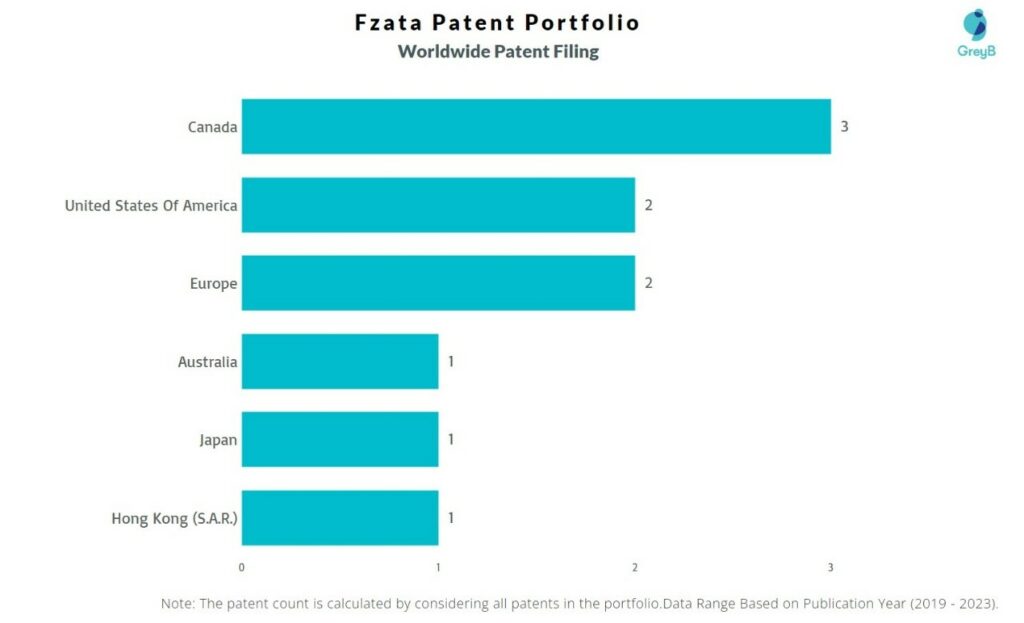
Countries in which Fzata Filed Patents
| Country | Patent |
| Canada | 3 |
| United States Of America | 2 |
| Europe | 2 |
| Australia | 1 |
| Japan | 1 |
| Hong Kong (S.A.R.) | 1 |
Where are Research Centers of Fzata Patents Located?
The Research Centers of Fzata Patents is the United States of America.
List of Fzata patents
| Fzata Patents | Title |
| US20240011985A1 | Neutralized Antibody And Method Of Use Thereof |
| US20220235120A1 | Humanized Tetra-Specific Octavalent Antibody Against Clostridium Difficile Toxin A And B |
| EP4232818A1 | Neutralized Antibody And Method Of Use Thereof |
| EP3976094A4 | Humanized Tetra-Specific Octavalent Antibody Against Clostridium Difficile Toxin A And B |
| CA3221887A1 | Genetically-Modified Saccharomyces Yeast Strains As Preventative And Therapeutic Agents |
| CA3196256A1 | Neutralized Antibody And Method Of Use Thereof |
| CA3142602A1 | Humanized Tetra-Specific Octavalent Antibody Against Clostridium Difficile Toxin A And B |
| JP2022535264A5 | Humanized Quadrispecific Octavalent Antibody Against Clostridium Difficile A-Toxin And B-Toxin |
| HK40072792A | Humanized Tetra-Specific Octavalent Antibody Against Clostridium Difficile Toxin A And B |
| AU2020287324A1 | Humanized Tetra-Specific Octavalent Antibody Against Clostridium Difficile Toxin A And B |
| WO2022261455A3 | Genetically-Modified Saccharomyces Yeast Strains As Preventative And Therapeutic Agents |
| WO2022087021A1 | Neutralized Antibody And Method Of Use Thereof |
| WO2020247500A1 | Humanized Tetra-Specific Octavalent Antibody Against Clostridium Difficile Toxin A And B |
What Technologies are Covered by Fzata?

The chart below distributes patents filed by Fzata
