Juno Diagnostics has a total of 50 patents globally, out of which 1 have been granted. Of these 50 patents, more than 70% patents are active. The United States of America is where Juno Diagnostics has filed the maximum number of patents, followed by Europe and Australia. Parallelly, United States of America seems to be the main focused R&D centre and also is the origin country of Juno Diagnostics.
Juno Diagnostics was founded in 2017. Juno Therapeutics is a biotechnology firm in the clinical stage of business. The company’s main objectives include the development of cancer immunotherapies and other research services.
Do read about some of the most popular patents of Juno Diagnostics which have been covered by us in this article and also you can find Juno Diagnostics patents information, the worldwide patent filing activity and its patent filing trend over the years, and many other stats over Juno Diagnostics patent portfolio.
How many patents does Juno Diagnostics have?
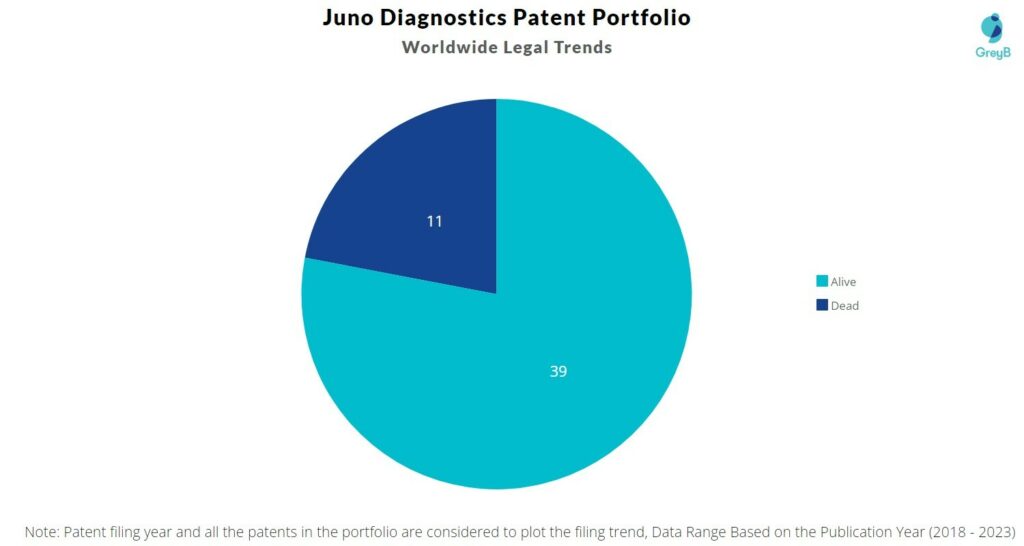
Juno Diagnostics has a total of 50 patents globally. These patents belong to 6 unique patent families. Out of 50 patents, 39 patents are active.
How Many Patents did Juno Diagnostics File Every Year?
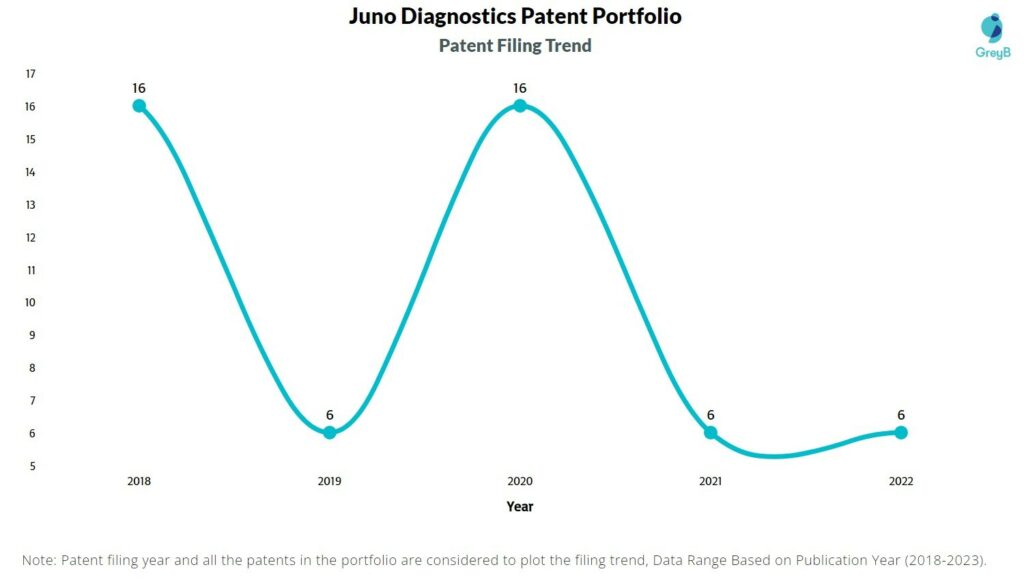
Are you wondering why there is a drop in patent filing for the last two years? It is because a patent application can take up to 18 months to get published. Certainly, it doesn’t suggest a decrease in the patent filing.
| Year of Patents Filing or Grant | Juno Diagnostics Applications Filed | Juno Diagnostics Patents Granted |
| 2022 | 6 | 1 |
| 2021 | 6 | – |
| 2020 | 16 | – |
| 2019 | 6 | – |
| 2018 | 16 | – |
How many Juno Diagnostics patents are Alive/Dead?
Worldwide Patents
How Many Patents did Juno Diagnostics File in Different Countries?
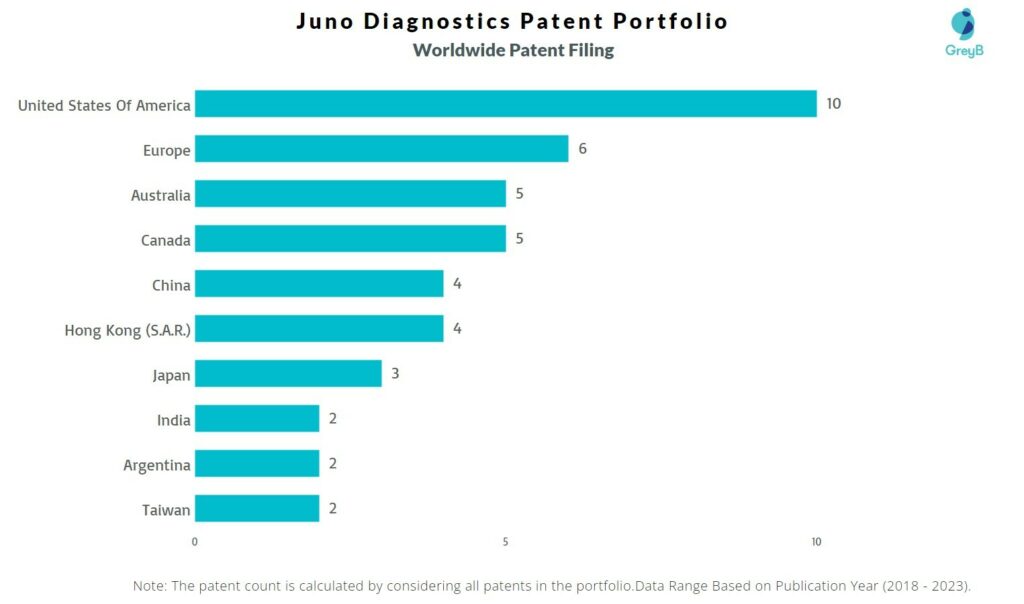
Countries in which Juno Diagnostics Filed Patents
| Country | Patent |
| United States Of America | 10 |
| Europe | 6 |
| Australia | 5 |
| Canada | 5 |
| China | 4 |
| Hong Kong (S.A.R.) | 4 |
| Japan | 3 |
| India | 2 |
| Argentina | 2 |
| Taiwan | 2 |
Where are Research Centers of Juno Diagnostics Patents Located?
The Research Center for Juno Diagnostics is located in United States of America.
10 Best Juno Diagnostics Patents
US20210020314A1 is the most popular patent in the Juno Diagnostics portfolio. It has received 9 citations so far from companies like Neuraville, Freenome Holdings and Sysmex Corporation.
Below is the list of 10 most cited patents of Juno Diagnostics:
| Publication Number | Citation Count |
| US20210020314A1 | 9 |
| WO2020198312A1 | 7 |
| WO2020198511A1 | 7 |
| WO2019067567A1 | 7 |
| CN112203648A | 4 |
| US20200263167A1 | 4 |
| WO2019084489A1 | 3 |
| US20220170915A1 | 2 |
| US20220162591A1 | 1 |
| US20200299677A1 | 1 |
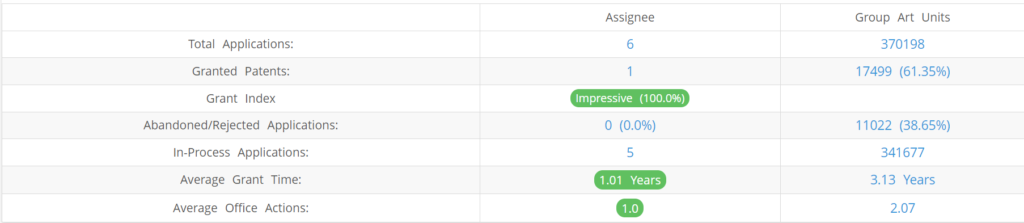
What Percentage of Juno Diagnostics US Patent Applications Were Granted?
Juno Diagnostics (Excluding its subsidiaries) has filed 6 patent applications at USPTO so far (Excluding Design and PCT applications). Out of these 1 have been granted leading to a grant rate of 100%.
Below are the key stats of Juno Diagnostics patent prosecution at the USPTO.
Which Law Firms Filed Most US Patents for Juno Diagnostics?
| Law Firm | Total Applications | Success Rate |
| Wilson Sonsini Goodrich & Rosati | 6 | 100% |
List of Juno Diagnostics patents
| Juno Diagnostics Patents | Title |
| US11525134B2 | Devices, Systems And Methods For Ultra-Low Volume Liquid Biopsy |
| US20230051179A1 | Devices, Systems And Methods For Ultra-Low Volume Liquid Biopsy |
| US20220404353A1 | Systems And Devices For Sample Preparation And Analyte Detection |
| US20220380752A1 | Devices And Methods For Extracting Blood Plasma |
| US20220170915A1 | Digital Health Ecosystem |
| US20220162591A1 | Optimized Ultra-Low Volume Liquid Biopsy Methods, Systems, And Devices |
| US20210020314A1 | Deep Learning-Based Methods, Devices, And Systems For Prenatal Testing |
| US20200299677A1 | Devices, Systems And Methods For Ultra-Low Volume Liquid Biopsy |
| EP4065730A4 | Systems And Devices For Sample Preparation And Analyte Detection |
| EP3947672A4 | Optimized Ultra-Low Volume Liquid Biopsy Methods, Systems, And Devices |
| EP3773534A4 | Deep Learning-Based Methods, Devices, And Systems For Prenatal Testing |
| EP3700423A4 | Devices, Systems And Methods For Ultra-Low Volume Liquid Biopsy |
| EP3688158A4 | Devices, Systems And Methods For Biomarker Analysis |
| CN113906146A | Optimized Ultra-Low Volume Liquid Biopsy Methods, Systems, And Devices |
| CN112203648A | Deep Learning-Based Methods, Devices, And Systems For Prenatal Testing |
| CN111526793A | Devices, Systems And Methods For Ultra-Low Volume Liquid Biopsy |
| CN111406107A | Devices, Systems And Methods For Biomarker Analysis |
| AU2020394370A1 | Systems And Devices For Sample Preparation And Analyte Detection |
| AU2020245532A1 | Optimized Ultra-Low Volume Liquid Biopsy Methods, Systems, And Devices |
| CA3162239A1 | Systems And Devices For Sample Preparation And Analyte Detection |
| AU2019244115A1 | Deep Learning-Based Methods, Devices, And Systems For Prenatal Testing |
| CA3134941A1 | Optimized Ultra-Low Volume Liquid Biopsy Methods, Systems, And Devices |
| AU2018355575A1 | Devices, Systems And Methods For Ultra-Low Volume Liquid Biopsy |
| AU2018342449A1 | Devices, Systems And Methods For Biomarker Analysis |
| CA3095030A1 | Deep Learning-Based Methods, Devices, And Systems For Prenatal Testing |
| CA3080117A1 | Devices, Systems And Methods For Ultra-Low Volume Liquid Biopsy |
| CA3076809A1 | Devices, Systems And Methods For Biomarker Analysis |
| HK40067251A | Optimized Ultra-Low Volume Liquid Biopsy Methods, Systems, And Devices |
| HK40038537A | Deep Learning-Based Methods, Devices, And Systems For Prenatal Testing |
| HK40036638A | Devices, Systems And Methods For Ultra-Low Volume Liquid Biopsy |
| HK40035115A | Devices, Systems And Methods For Biomarker Analysis |
| AR125967A1 | Devices, Systems And Methods For Ultra-Low Volume Liquid Biopsy |
| JP2022525953A5 | Optimized Ultratrace Liquid Biopsy Methods, Systems, And Devices |
| TW202313970A | Devices And Methods For Extracting Blood Plasma |
| IN202117042324A | Optimized Ultra-Low Volume Liquid Biopsy Methods, Systems, And Devices |
| JP2021500883A5 | Devices, Systems And Methods For Ultra-Trace Liquid Biopsy |
| IN202017017971A | Devices, Systems And Methods For Ultra-Low Volume Liquid Biopsy |
| AR113802A1 | Devices, Systems And Methods For Ultra Low Volume Liquid Biopsy |
| TW201923090A | Devices, Systems And Methods For Ultra-Low Volume Liquid Biopsy |
| US20220119801A1 | Devices, Systems And Methods For Biomarker Analysis |
| US20200263167A1 | Devices, Systems And Methods For Biomarker Analysis |
| EP3946053A4 | Digital Health Ecosystem |
| WO2022251346A1 | Devices And Methods For Extracting Blood Plasma |
| WO2021108266A1 | Systems And Devices For Sample Preparation And Analyte Detection |
| WO2020198312A1 | Optimized Ultra-Low Volume Liquid Biopsy Methods, Systems, And Devices |
| WO2020198511A1 | Digital Health Ecosystem |
| WO2019191319A1 | Deep Learning-Based Methods, Devices, And Systems For Prenatal Testing |
| WO2019084489A1 | Devices, Systems And Methods For Ultra-Low Volume Liquid Biopsy |
| WO2019067567A1 | Devices, Systems And Methods For Biomarker Analysis |
| JP2020534873A5 | Devices, Systems And Methods For Biomarker Analysis |
What are Juno Diagnostics key innovation segments?
What Technologies are Covered by Juno Diagnostics?

The chart below distributes patents filed by Juno Diagnostics
