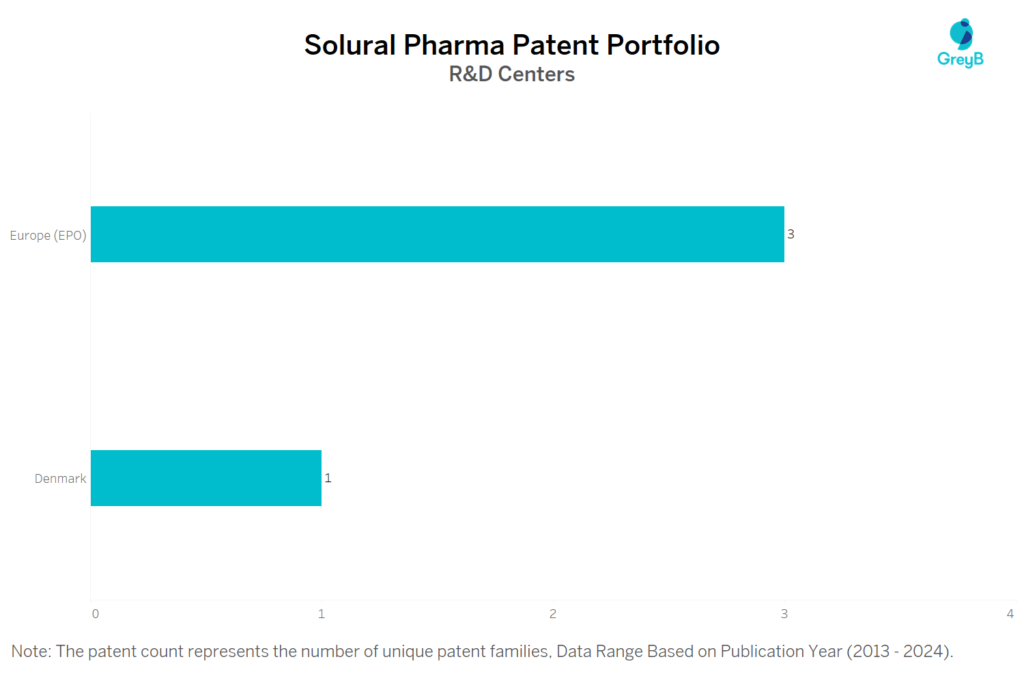
Solural Pharma has a total of 42 patents globally, out of which 24 have been granted. Of these 42 patents, more than 76% patents are active. Japan is where Solural Pharma has filed the maximum number of patents. Parallelly, Europe (EPO) seems to be the main focused R&D centre and also is the origin country of Solural Pharma.
Solural Pharma was founded in 2012. Solural Pharma specializes in pharmaceutical development services aimed at treating significant medical conditions. Their research and development prioritizes cutting-edge technology to facilitate drug discovery, thereby aiding patients in achieving early recovery through orally-administered drugs with enhanced solubility.
Do read about some of the most popular patents of Solural Pharma which have been covered by us in this article and also you can find Solural Pharma patents information, the worldwide patent filing activity and its patent filing trend over the years, and many other stats over Solural Pharma patent portfolio.
How many patents does Solural Pharma have?
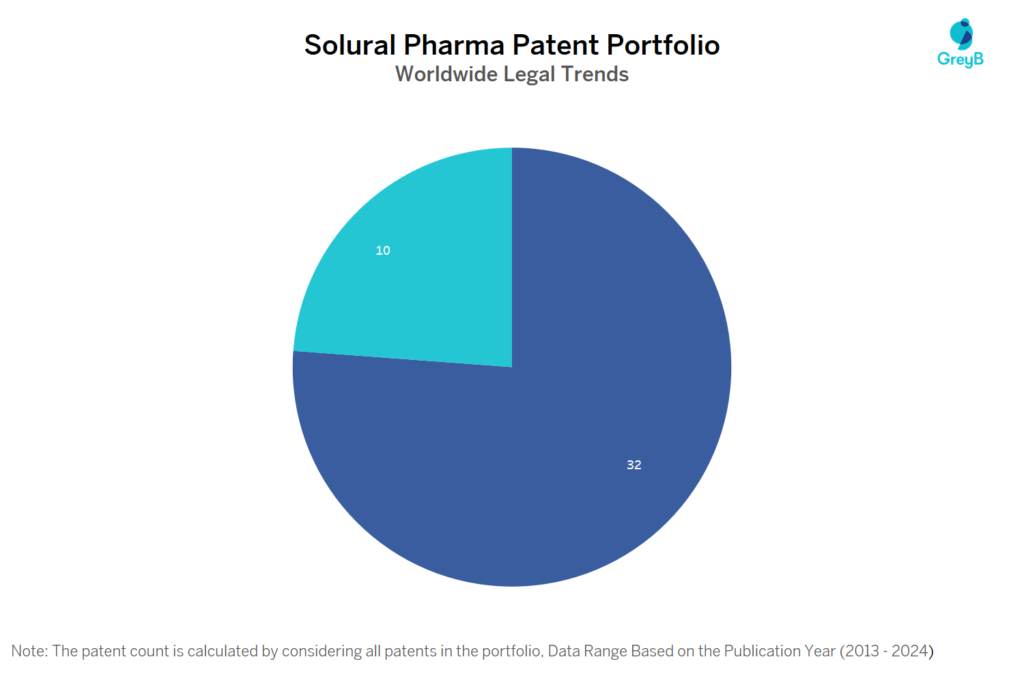
Solural Pharma has a total of 42 patents globally. These patents belong to 4 unique patent families. Out of 42 patents, 32 patents are active.
How Many Patents did Solural Pharma File Every Year?
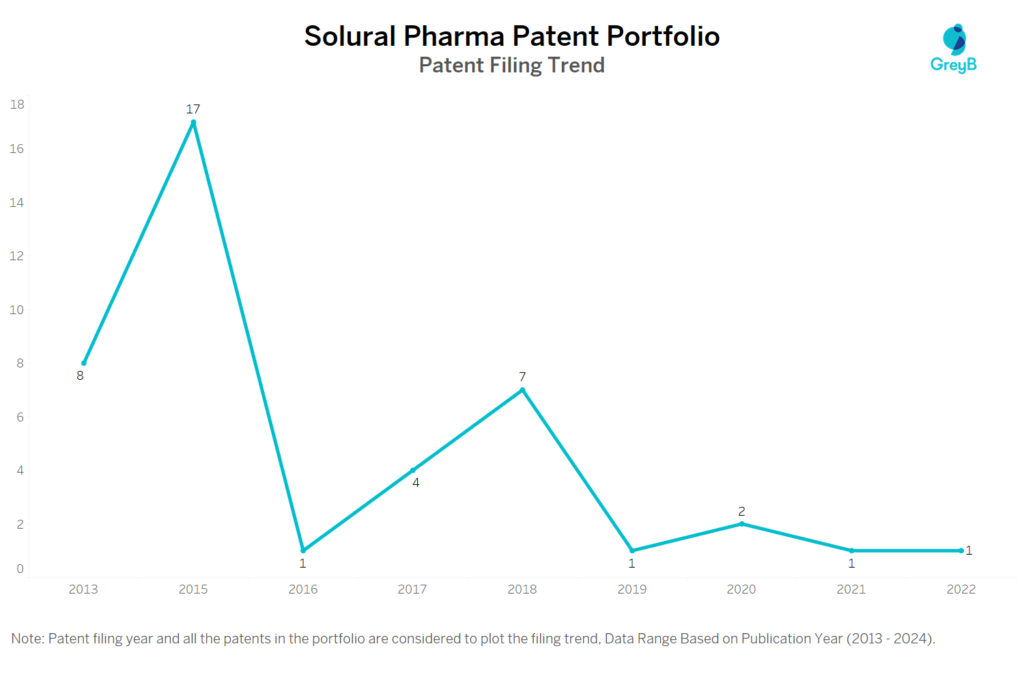
Are you wondering why there is a drop in patent filing for the last two years? It is because a patent application can take up to 18 months to get published. Certainly, it doesn’t suggest a decrease in the patent filing.
| Year of Patents Filing or Grant | Solural Pharma Applications Filed | Solural Pharma Patents Granted |
| 2023 | – | 4 |
| 2022 | 1 | 3 |
| 2021 | 1 | 3 |
| 2020 | 2 | 7 |
| 2019 | 1 | – |
| 2018 | 7 | 1 |
| 2017 | 4 | 6 |
| 2016 | 1 | – |
| 2015 | 17 | – |
| 2013 | 8 | – |
How many Solural Pharma patents are Alive/Dead?
Worldwide Patents
How Many Patents did Solural Pharma File in Different Countries?
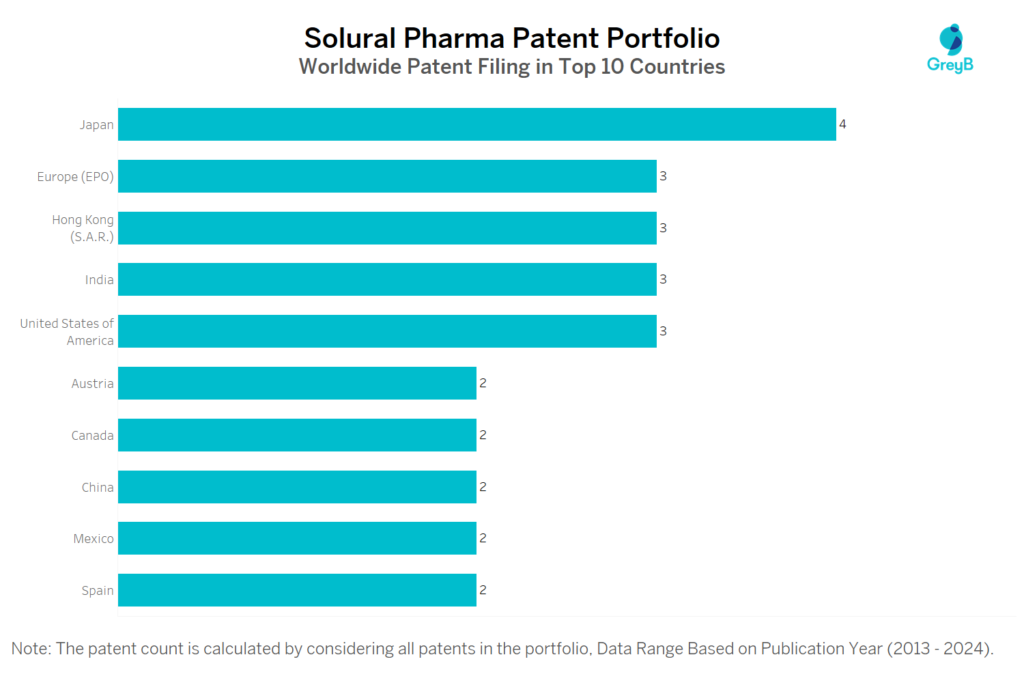
Countries in which Solural Pharma Filed Patents
| Country | Patent |
| Japan | 4 |
| United States of America | 3 |
| India | 3 |
| Europe (EPO) | 3 |
| Hong Kong (S.A.R.) | 3 |
| China | 2 |
| Spain | 2 |
| Mexico | 2 |
| Austria | 2 |
| Canada | 2 |
| Poland | 1 |
| Eurasian Patent Organization | 1 |
| Brazil | 1 |
| Singapore | 1 |
| Norway | 1 |
| Denmark | 1 |
| Portugal | 1 |
| Italy | 1 |
| Germany | 1 |
| Israel | 1 |
| Australia | 1 |
| Hungary | 1 |
Where are Research Centers of Solural Pharma Patents Located?
List of Solural Pharma patents
| Solural Pharma Patents | Title |
| US11819482B2 | Composition Comprising Suplatast Tosilate |
| US9682148B2 | Solid Oral Dosage Form Of Testosterone Derivative |
| EP2934591B1 | Solid Oral Dosage Form Of Testosterone Derivative |
| EP3157508B1 | Solid Oral Dosage Form Of Lipophilic Compounds |
| EP3675831A1 | Composition Comprising Suplatast Tosilate |
| CN111032021A | Composition Comprising Suplatast Tosilate |
| DE602013024910T2 | Solid Oral Dosage Form Of A Testosterone Derivative |
| AT1342646T | Solid Oral Dosage Form Of Lipophilic Compounds |
| IT202100011963T2 | Solid Oral Dosage Form Of Lipophilic Compounds |
| ES2638981T3 | Solid Oral Dosage Form Of Testosterone Derivative |
| ES2851332T3 | Solid Oral Dosage Form Of Lipophilic Compounds |
| BR112016029271B1 | Solid Oral Dosage Form Of Lipophilic Compounds |
| CA2931086C | Solid Oral Dosage Form Of Testosterone Derivative |
| DK3157508T3 | Solid Oral Dosage Form Of Lipofilic Compounds |
| EA036836B1 | Pharmaceutical Composition For Providing Lymphatic Absorption Of Lipophilic Compounds In Fed And In Fasted State |
| IL249238B | Solid Oral Dosage Form Of Lipophilic Compounds |
| IN389113B | Solid Oral Dosage Form Of Lipophilic Compounds |
| JP6723166B2 | Solid Oral Dosage Forms Of Lipophilic Compounds |
| JP7071420B2 | Solid Oral Dosage Forms Of Lipophilic Compounds |
| JP7330948B2 | Compositions Containing Suplatast Tosylate |
| MX360186B | Solid Oral Dosage Form Of Testosterone Derivative. |
| MX377352B | Solid Oral Dose Form Of Lipophilic Compounds. |
| NO3157508B1 | Solid Oral Dosage Form Of Lipophilic Compounds |
| PL3157508T3 | Solid Oral Dosage Form Of Lipophilic Compounds |
| CA3073866A1 | Composition Comprising Suplatast Tosilate |
| HK1235016A | Solid Oral Dosage Form Of Lipophilic Compounds |
| HK40034147A | Composition Comprising Suplatast Tosilate |
| HK40078012A | Solid Oral Dosage Form Of Lipophilic Compounds |
| HUE054467T2 | Solid Oral Dosage Form Of Lipophilic Compounds |
| JP2022120040A | Pulsatile Drug Delivery System For Treating Morning Immobility |
| PT3157508T | Solid Oral Dosage Form Of Lipophilic Compounds |
| SG11201609352TB | Solid Oral Dosage Form Of Lipophilic Compounds |
| US20140179655A1 | Solid Oral Dosage Form Of Testosterone Derivative |
| CN107073127A | Solid Oral Dosage Form Of Lipophilic Compounds |
| WO2014096139A1 | Solid Oral Dosage Form Of Testosterone Derivative |
| WO2015193380A3 | Solid Oral Dosage Form Of Lipophilic Compounds |
| WO2018011181A1 | Pulsatile Drug Delivery System For Treating Morning Akinesia |
| WO2019042995A1 | Composition Comprising Suplatast Tosilate |
| AT916108T | Solid Oral Dosage Form Of A Testosterone Derivative |
| AU2018322756B2 | Composition Comprising Suplatast Tosilate |
| IN201927001211A | Pulsatile Drug Delivery System For Treating Morning Akinesia |
| IN202017010343A | Composition Comprising Suplatast Tosilate |
What are Solural Pharma key innovation segments?
What Technologies are Covered by Solural Pharma?

The chart below distributes patents filed by Solural Pharma in different countries on the basis of the technology protected in patents. It also represents the markets where Solural Pharma thinks it’s important to protect particular technology inventions.

R&D Focus: How has Solural Pharma search focus changed over the years?

EXCLUSIVE INSIGHTS COMING SOON!


