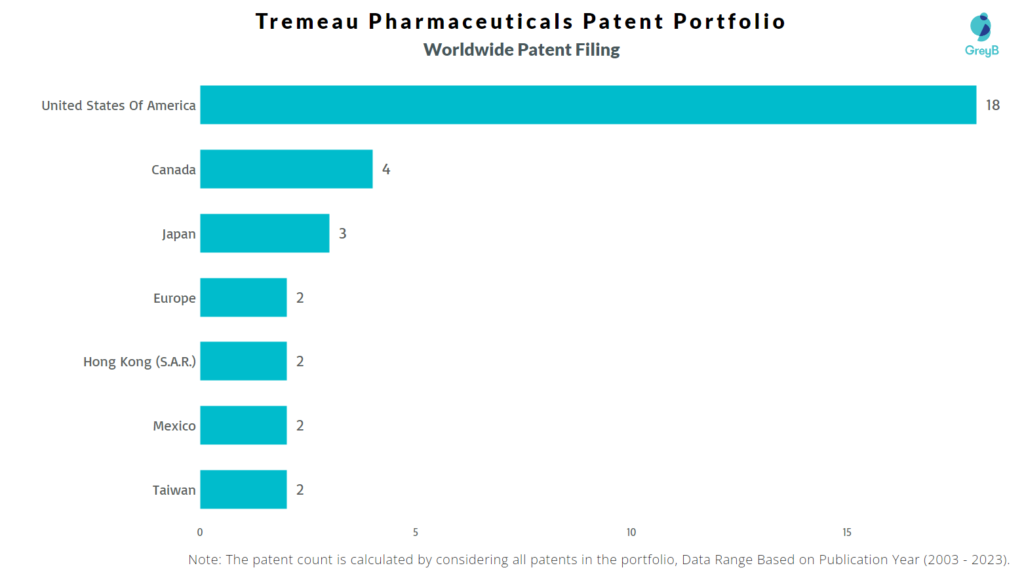
Tremeau Pharmaceuticals has a total of 39 patents globally, out of which 7 have been granted. Of these 39 patents, more than 74% patents are active. The USA is where Tremeau Pharmaceuticals has filed the maximum number of patents, followed by Canada and Japan. Parallelly, the USA seems to be the main focused R&D center of Tremeau Pharmaceuticals and is also the origin country of the firm.
Tremeau Pharmaceuticals was founded in the year 2016 by Bradford Sippy and Mark Corrigan. The company operates as a pharmaceutical firm. It focuses on commercializing non-opioid pain therapies targeting serious rare diseases impacted by the cyclooxygenase and prostanoid pathways.
Do read about some of the most popular patents of Tremeau Pharmaceuticals which have been covered by us in this article and also you can find Tremeau Pharmaceuticals patents information, the worldwide patent filing activity and its patent filing trend over the years, and many other stats over Tremeau Pharmaceuticals patent portfolio.
How many patents does Tremeau Pharmaceuticals have?
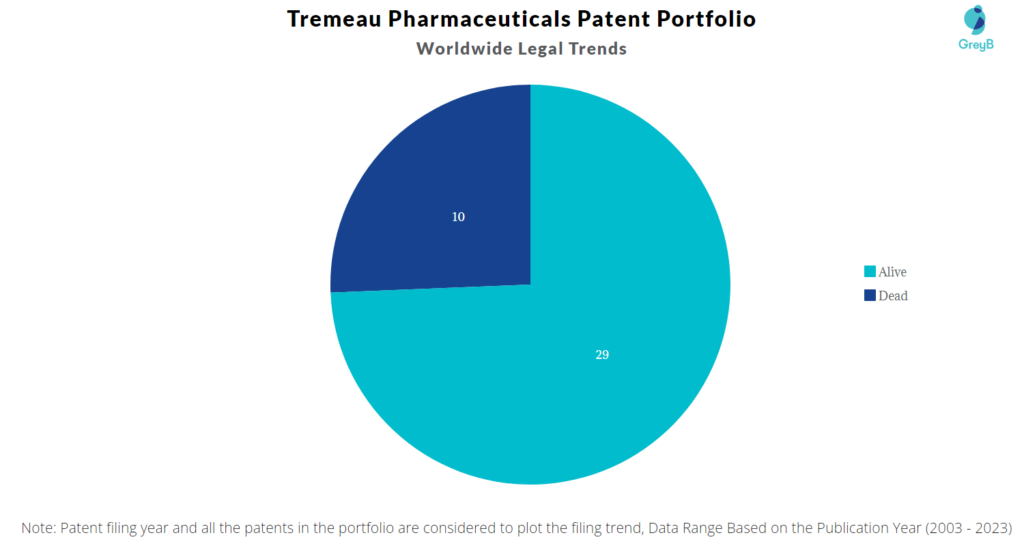
Tremeau Pharmaceuticals has a total of 39 patents globally. These patents belong to 8 unique patent families. Out of 39 patents, 29 patents are active.
How Many Patents did Tremeau Pharmaceuticals File Every Year?
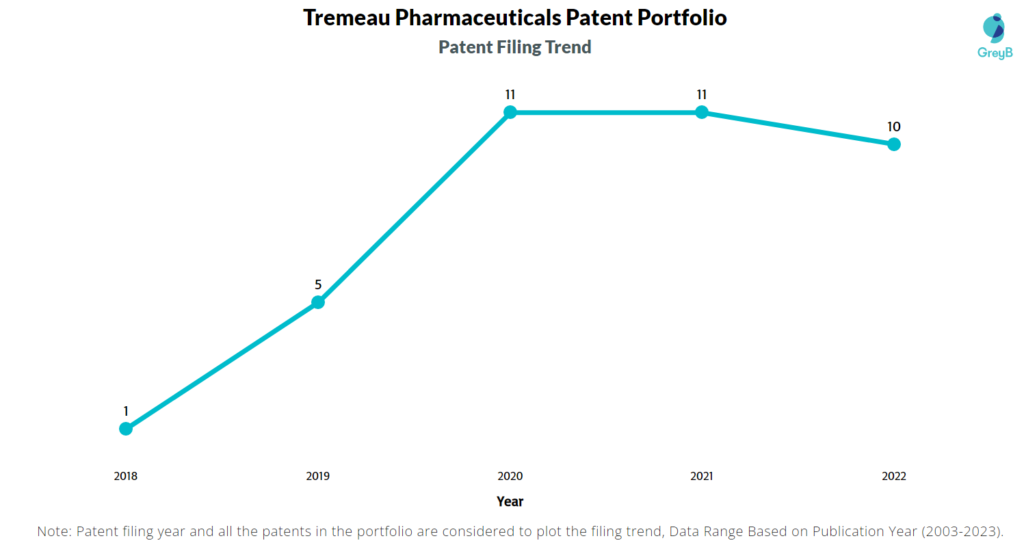
Are you wondering why there is a drop in patent filing for the last two years? It is because a patent application can take up to 18 months to get published. Certainly, it doesn’t suggest a decrease in the patent filing.
| Year of Patents Filing or Grant | Tremeau Pharmaceuticals Applications Filed | Tremeau Pharmaceuticals Patents Granted |
| 2022 | 10 | – |
| 2021 | 11 | 3 |
| 2020 | 11 | – |
| 2019 | 5 | – |
| 2018 | 1 | – |
| 2017 | – | – |
| 2016 | – | – |
How many Tremeau Pharmaceuticals patents are Alive/Dead?
Worldwide Patents
How Many Patents did Tremeau Pharmaceuticals File in Different Countries?
Where are Research Centres of Tremeau Pharmaceuticals Patents Located?
The R&D Centres of Tremeau Pharmaceuticals is in the USA.
10 Best Tremeau Pharmaceuticals Patents
US10987337B2 is the most popular patent in the Tremeau Pharmaceuticals portfolio. It has received 7 citations so far.
Below is the list of 10 most cited patents of Tremeau Pharmaceuticals:
| Publication Number | Citation Count |
| US10987337B2 | 7 |
| WO2020106522A1 | 7 |
| US10945992B1 | 5 |
| US20220047542A1 | 3 |
| US20210169845A1 | 3 |
| US20210137878A1 | 3 |
| US11161833B1 | 2 |
| US11576890B2 | 1 |
| US20220378736A1 | 1 |
| US20190083405A1 | 1 |
List of Tremeau Pharmaceuticals
| Tremeau Pharmaceuticals Patents | Title |
| US11617735B2 | Purified Forms Of Rofecoxib, Methods Of Manufacture And Use |
| US11576890B2 | Purified Forms Of Rofecoxib, Methods Of Manufacture And Use |
| US11559509B2 | Purified Forms Of Rofecoxib, Methods Of Manufacture And Use |
| US11161833B1 | Deuterated Etoricoxib, Methods Of Manufacture, And Use Thereof |
| US10987337B2 | Purified Forms Of Rofecoxib, Methods Of Manufacture And Use |
| US10945992B1 | Dosage Forms Of Rofecoxib And Related Methods |
| US20220409575A1 | Dosage Forms Of Rofecoxib And Related Methods |
| US20220378736A1 | Purified Forms Of Rofecoxib, Methods Of Manufacture And Use |
| US20220332696A1 | Deuterated Etoricoxib, Methods Of Manufacture, And Use Thereof |
| US20220273609A1 | Treatment Of Viral Hemorrhagic Fevers With Etoricoxib |
| US20220125773A1 | Aqueous Formulations Of Water Insoluble Cox 2 Inhibitors |
| US20210244672A1 | Ingestible Product And A Method Of Using The Same |
| US20200323813A1 | Treatment Of Viral Hemorrhagic Fevers With Cox 2 Selective Non Steroidal Anti Inflammatory Drugs |
| EP4058012A1 | Novel Dosage Forms Of Rofecoxib And Related Methods |
| EP3883562A4 | Purified Forms Of Rofecoxib, Methods Of Manufacture And Use |
| WO2023059698A1 | Methods And Compositions For Treating Von Willebrand’S Migraine Disorder |
| CA3214485A1 | Deuterated Etoricoxib, Methods Of Manufacture, And Use Thereof |
| CA3200132A1 | Aqueous Formulations Of Water Insoluble Cox 2 Inhibitors |
| CA3155900A1 | Novel Dosage Forms Of Rofecoxib And Related Methods |
| CA3119728A1 | Purified Forms Of Rofecoxib, Methods Of Manufacture And Use |
| HK40064694A | Purified Forms Of Rofecoxib, Methods Of Manufacture And Use |
| HK40062093A | Purified Forms Of Rofecoxib, Methods Of Manufacture And Use |
| MX2022005813A | Novel Dosage Forms Of Rofecoxib And Related Methods. |
| MX2021005967A | Purified Forms Of Rofecoxib, Methods Of Manufacture And Use. |
| JP7291220B2 | Purified Forms Of Rofecoxib, Methods Of Manufacture And Uses |
| JP2023124871A | Purified Forms Of Rofecoxib, Methods Of Manufacture And Uses |
| JP2023502941A | Novel Dosage Forms Of Rofecoxib And Associated Methods |
| TW202304873A | Deuterated Etoricoxib, Methods Of Manufacture, And Use Thereof |
| TW202128144A | Novel Dosage Forms Of Rofecoxib And Related Methods |
| US20220047542A1 | Novel Dosage Forms Of Rofecoxib And Related Methods |
| US20210169845A1 | Novel Dosage Forms Of Rofecoxib And Related Methods |
| US20210137878A1 | Novel Dosage Forms Of Rofecoxib And Related Methods |
| US20200323830A1 | Treatment Of Viral Hemorrhagic Fevers With Etoricoxib |
| US20190083405A1 | Ingestible Product And A Method Of Using The Same |
| WO2022093978A1 | Aqueous Formulations Of Water Insoluble Cox 2 Inhibitors |
| WO2021097038A1 | Novel Dosage Forms Of Rofecoxib And Related Methods |
| WO2020210373A1 | Treatment Of Viral Hemorrhagic Fevers With Etoricoxib |
| WO2020210341A1 | Treatment Of Viral Hemorrhagic Fevers With Cox 2 Selective Non Steroidal Antiinflammatory Drugs |
| WO2020106522A1 | Purified Forms Of Rofecoxib, Methods Of Manufacture And Use |
What are Tremeau Pharmaceuticals’ key innovation segments?
What Technologies are Covered byTremeau Pharmaceuticals?

The chart below distributes patents filed by Tremeau Pharmaceuticals
